|
Synonyms: compound 43d [PMID: 31721572] | SAR-439859 | SAR439859
Compound class:
Synthetic organic
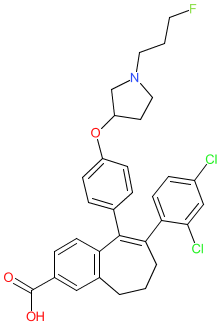
Comment: Amcenestrant (SAR439859) is a selective estrogen receptor degrader (SERD) [1]. It was designed (by Sanofi) to have improved pharmacokinetic properties compared to the existing clinically used SERD, fulvestrant. An X-ray structure of SAR439859 in complex with the ERα ligand binding domain indicates that the ligand occupies the orthosteric estradiol binding cavity.
|
|
|||||||||||||||||||||||||||||||||||
| Bioactivity Comments |
| SAR439859 degrades ERα in MCF-7 cells (by fluorescence detection following 4h treatment) with an EC50 of 0.2 nM, and an efficacy of 98% compared to fulvestrant control [1]. Orally dosed SAR439859 exhibits anti-tumour activity in xenograft murine models of ER+ve breast cancer, and is well tolerated. Direct agonist/antagonist activity of SAR439859 at the ERα is not reported in the discovery article. |
| Selectivity at nuclear hormone receptors | ||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | |||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||