GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
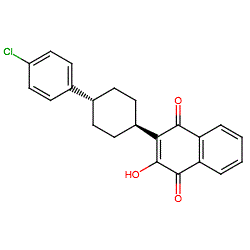
Synonyms: 566C80 | Mepron® | Wellvone®
atovaquone is an approved drug (FDA (1992))
Compound class:
Synthetic organic
Comment: Atovaquone is a synthetic hydroxynaphthoquinone with broad-spectrum antiprotozoal activity. It is an antimalarial drug and used in combination with proguanil.
Note that we have drawn the molecule as specified by the INN record and ChEMBL entry listed on this summary page. The structure for PubChem CID 74989 (link in table below) is shown without specified stereochemistry (InChi Key BSJMWHQBCZFXBR-UHFFFAOYSA-N). Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Atovaquone is used in combination with proguanil in both the treatment of uncomplicated malaria caused by P. falciparum and in malaria prophylaxis [1-2]. The use of atovaquone as an antimalarial monotherapy is not indicated because of concerns regarding recrudescence rates and parasite resistance [7]. Other uses of atovaquone are as a combined therapy with azithromycin for the treatment of babesiosis [6] and as a monotherapy for the prevention and treatment of mild-to moderate pneumonia caused by P. jirovecii (PCP) in individuals who are sensitive to trimethoprim-sulfamethoazole treatment [5]. |
Mechanism Of Action and Pharmacodynamic Effects  |
| The mechanism of action of atovaquone has been studied most extensively in the Plasmodium parasite. The compound inhibits the cytochrome bc1 complex of the mitochondrial electron-transfer chain, with a subsequent collapse of the mitochondrial membrane potential [9-10]. An active cytochrome bc1 complex is necessary for the biosynthesis of pyrimidines and disruption of this pathway is lethal to the parasite [8]. |
External links  |
|
For extended ADME data see the following: Drugs.com |