GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
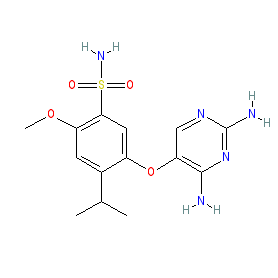
Synonyms: AF-219 | AF219 | Lyfnua® | MK7264 | MK‐7264
gefapixant is an approved drug (Japan (2022), EMA (2023))
Compound class:
Synthetic organic
Comment: Gefapixant (MK‐7264, and formerly AF-219) is a P2X3 and P2X2/3 receptor antagonist. Experimental results have shown that gefapixant acts as a reversible allosteric antagonist, that exhibits preferential activity at closed channels.
Studies in rodent models have provided evidence that supports investigation of gefapixant as a novel treatment for chronic sensitisation conditions [7]. Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Abdulqawi R, Dockry R, Holt K, Layton G, McCarthy BG, Ford AP, Smith JA. (2015)
P2X3 receptor antagonist (AF-219) in refractory chronic cough: a randomised, double-blind, placebo-controlled phase 2 study. Lancet, 385 (9974): 1198-205. [PMID:25467586] |
|
2. Ford AP, Undem BJ. (2013)
The therapeutic promise of ATP antagonism at P2X3 receptors in respiratory and urological disorders. Front Cell Neurosci, 7: 267. [PMID:24391544] |
|
3. Jacobson KA, Müller CE. (2016)
Medicinal chemistry of adenosine, P2Y and P2X receptors. Neuropharmacology, 104: 31-49. [PMID:26686393] |
|
4. Markham A. (2022)
Gefapixant: First Approval. Drugs, 82 (6): 691-695. [PMID:35347635] |
|
5. McGarvey LP, Birring SS, Morice AH, Dicpinigaitis PV, Pavord ID, Schelfhout J, Nguyen AM, Li Q, Tzontcheva A, Iskold B et al.. (2022)
Efficacy and safety of gefapixant, a P2X3 receptor antagonist, in refractory chronic cough and unexplained chronic cough (COUGH-1 and COUGH-2): results from two double-blind, randomised, parallel-group, placebo-controlled, phase 3 trials. Lancet, 399 (10328): 909-923. [PMID:35248186] |
|
6. Ochoa-Cortes F, Liñán-Rico A, Jacobson KA, Christofi FL. (2014)
Potential for developing purinergic drugs for gastrointestinal diseases. Inflamm Bowel Dis, 20 (7): 1259-87. [PMID:24859298] |
|
7. Richards D, Gever JR, Ford AP, Fountain SJ. (2019)
Action of MK-7264 (gefapixant) at human P2X3 and P2X2/3 receptors and in vivo efficacy in models of sensitisation. Br J Pharmacol, 176 (13): 2279-2291. [PMID:30927255] |
|
8. Smith JA, Kitt MM, Butera P, Smith SA, Li Y, Xu ZJ, Holt K, Sen S, Sher MR, Ford AP. (2020)
Gefapixant in two randomised dose-escalation studies in chronic cough. Eur Respir J, 55 (3). [PMID:31949115] |