GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
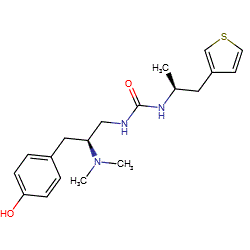
Synonyms: (S,S)-1-[2-(dimethylamino)-3-(4-hydroxyphenyl)propyl]-3-(1-thiophen-3-ylpropan-2-yl)urea | (S,S)-21 [PMID: 27533032]
Compound class:
Synthetic organic
Comment: PZM21 is a potent, selective μ opioid receptor agonist with bias towards G-protein (Gi) activation over β-arrestin 2 recruitment [1]. This provides a compound with reduced propensity to produce common opioid side effects whilst retaining analgesic activity. PZM21 is being used experimentally for its analgesic activity.
This compound is not in PubChem and its SMILES string does not retrieve any patent documents from SureChembl (late August 2016). Compare this with oliceridine, a chemically unrelated μ opioid receptor G-protein biased agonist. Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| Bioactivity Comments |
| In mouse experiments PZM21 produces significant analgesia but with markedly reduced side effects of constipation and respiratory depression compared to morphine [1]. PZM21 is selective for the μ opiod receptor over other GPCRs tested, and does not inhibit neurotransmitter transporters with high affinity [1]. It behaves as an antagonist of the κ opiod receptor (Ki 18nM). PZM21 inhibits hERG with IC50 2000-4000nM. |
| Selectivity at GPCRs | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||