GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
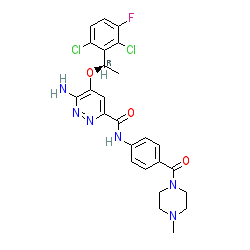
Synonyms: Ensacove® | Example 18 [WO2012048259] [3] | X-396
ensartinib is an approved drug (FDA (2024))
Compound class:
Synthetic organic
Comment: Ensartinib (X-396) is an orally available, investigational inhibitor of anaplastic lymphoma receptor tyrosine kinase (ALK). It blocks ALK-mediated signalling and inhibits proliferation in tumours harbouring ALK fusions and mutations [4].
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Ding L, Yuan X, Wang Y, Yang M, Wu P, Chen H, Yun Y, Shen Z, Ji D, Ma Y. (2024)
Ensartinib in the treatment of anaplastic lymphoma kinase-positive locally advanced or metastatic patients with lung squamous or adenosquamous carcinoma: A real-world, retrospective study. Asia Pac J Clin Oncol, 20 (6): 700-706. [PMID:38898784] |
|
2. Horn L, Wang Z, Wu G, Poddubskaya E, Mok T, Reck M, Wakelee H, Chiappori AA, Lee DH, Breder V et al.. (2021)
Ensartinib vs Crizotinib for Patients With Anaplastic Lymphoma Kinase-Positive Non-Small Cell Lung Cancer: A Randomized Clinical Trial. JAMA Oncol, 7 (11): 1617-1625. [PMID:34473194] |
|
3. Liang C. (2012)
Substituted pyridazine carboxamide compounds. Patent number: WO2012048259. Assignee: Xcovery Holding Company, Llc. Priority date: 08/10/2010. Publication date: 12/04/2012. |
|
4. Lovly CM, Heuckmann JM, de Stanchina E, Chen H, Thomas RK, Liang C, Pao W. (2011)
Insights into ALK-driven cancers revealed through development of novel ALK tyrosine kinase inhibitors. Cancer Res, 71 (14): 4920-31. [PMID:21613408] |
|
5. Singhi EK, Horn L. (2018)
Background and rationale of the eXalt3 trial investigating X-396 in the treatment of ALK+ non-small-cell lung cancer. Future Oncol, 14 (18): 1781-1787. [PMID:29506392] |
|
6. Xu H, Chen T, Zheng Y, Fang W. (2024)
Ensartinib rapidly relieves symptoms in ALK-positive patients with brain metastases. Asian J Surg, 47 (9): 3947-3949. [PMID:38749835] |
|
7. Zhao M, Shao T, Shao H, Zhou C, Tang W. (2024)
Identifying optimal ALK inhibitors in first- and second-line treatment of patients with advanced ALK-positive non-small-cell lung cancer: a systematic review and network meta-analysis. BMC Cancer, 24 (1): 186. [PMID:38331773] |