GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: K-17
thalidomide is an approved drug (FDA (1998), EMA (2008))
Compound class:
Synthetic organic
Comment: Thalidomide is principally an immunomodulatory drug. It inhibits synthesis of TNFα. Mechanistically, thalidomide binds to cereblon, and this complex recruits substrate proteins for degradation by the ubiquitin system. The lymphoid transcription factors Ikaros (IKZF1) and Aiolos (IKZF3) have been identified as substrates for thalidomide-bound cereblon. More recently another transcription factor, PLZF (ZBTB16), has been reported as a potential thalidomide/cereblon substrate [7]. Knockdown of Plfz induces skeletal abnormalities in chicken limbs, so thalidomide-targeted degradation of PLZF would be predicted to exhibit similar teratogenic effects.
SARS-CoV-2 and COVID-19: Thalidomide + low-dose glucocorticoid is being evaluated for efficacy in severe COVID-19 pneumonia (preprint available here https://www.preprints.org/manuscript/202002.0395/v1). An alternative approach is examining the combination of thalidomide + celecoxib (which targets NF-κB to suppress production of inflammatory cytokines; see preprint DOI: 10.13140/RG.2.2.26979.91689). Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
Classification  |
|
| Compound class | Synthetic organic |
| Ligand families/groups | PROTACs, molecular glues and other degraders |
| Approved drug? | Yes. EU EMA (2008) | US FDA (1998) |
| WHO Essential Medicine | WHO Essential Medicines List (EML) (24th List, 2025). Download PDF version. Click to view more information about the WHO Model Lists of Essential Medicines. |
IUPAC Name  |
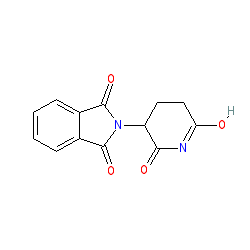
| 2-(6-hydroxy-2-oxo-2,3,4,5-tetrahydropyridin-3-yl)-2,3-dihydro-1H-isoindole-1,3-dione |
International Nonproprietary Names  |
|
| INN number | INN |
| 762 | thalidomide |
Synonyms  |
| K-17 |
Database Links  |
|
| Specialist databases | |
Reactome Drug

|
R-ALL-9681254 |
Reactome Reaction

|
R-HSA-9681169 |
| Other databases | |
| CAS Registry No. | 50-35-1 |
| ChEMBL Ligand | CHEMBL468 |
| DrugBank Ligand | DB01041 |
| DrugCentral Ligand | 2616 |
| GtoPdb PubChem SID | 178103899 |
| Immunopaedia Search | thalidomide |
| PubChem CID | 5426 |
| Search Google for chemical match using the InChIKey | UEJJHQNACJXSKW-UHFFFAOYSA-N |
| Search Google for chemicals with the same backbone | UEJJHQNACJXSKW |
| Search PubMed clinical trials | thalidomide |
| Search PubMed titles | thalidomide |
| Search PubMed titles/abstracts | thalidomide |
| UniChem Compound Search for chemical match using the InChIKey | UEJJHQNACJXSKW-UHFFFAOYSA-N |
| UniChem Connectivity Search for chemical match using the InChIKey | UEJJHQNACJXSKW-UHFFFAOYSA-N |
| Wikipedia | Thalidomide |
Product suppliers
View disclaimer
Sponsored products disclaimer
Product supplier links are provided as a service to assist in identifying commercial suppliers of reagents that are mentioned on the IUPHAR/BPS Guide to PHARMACOLOGY database website, and do not imply their endorsement by NC-IUPHAR.
Links are provided in return for sponsorship, used to fund improvements to this database. The sponsorship account is managed and audited by the University of Edinburgh, a charitable body registered in Scotland, SC005336. If you are interested in sponsoring the database, please contact us.
✖
Sponsored products disclaimer
Product supplier links are provided as a service to assist in identifying commercial suppliers of reagents that are mentioned on the IUPHAR/BPS Guide to PHARMACOLOGY database website, and do not imply their endorsement by NC-IUPHAR.
Links are provided in return for sponsorship, used to fund improvements to this database. The sponsorship account is managed and audited by the University of Edinburgh, a charitable body registered in Scotland, SC005336. If you are interested in sponsoring the database, please contact us.
✖|
Thalidomide (links to external site)
Cat. No. HY-14658 |