GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: Cortrosyn®
cosyntropin is an approved drug (FDA (1970))
Compound class:
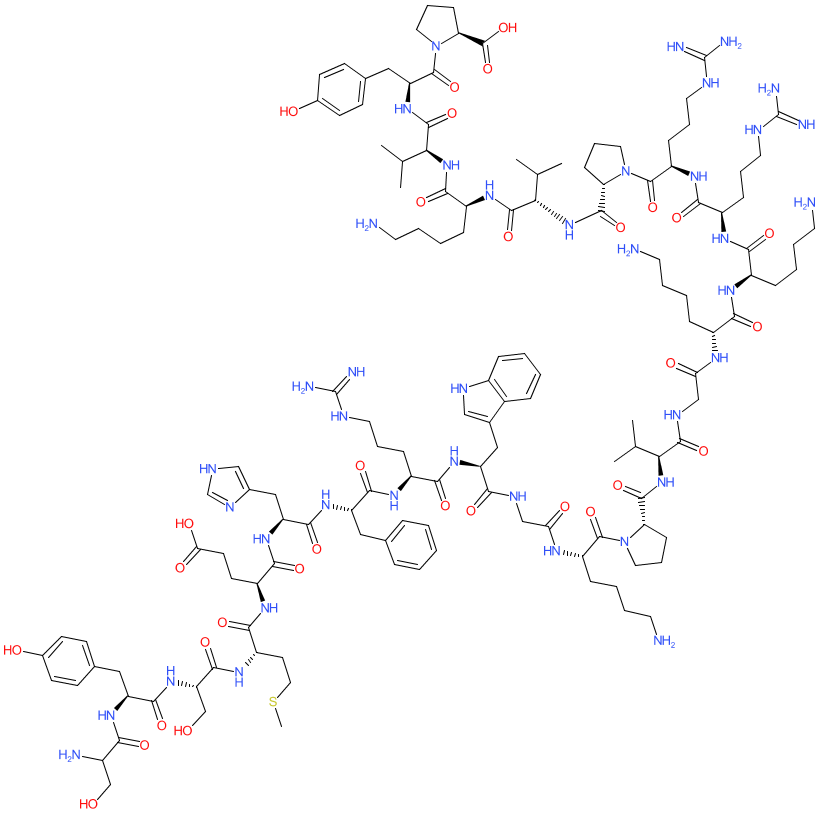
Peptide
Comment: This is a synthetic peptide forming the fully active N-terminal portion of cortocotropin. Like the endogenous peptide, cosyntropin stimulates the adrenal cortex to produce and secrete adrenocortical hormones.
|
|
|||||||||||||||||
For advanced searching click here to open chemical structure editor
Other Similar Sequences  |
|
| α-MSH {Sp: Human, Mouse, Rat} |
TargetsMC1 receptor; MC3 receptor; MC4 receptor; MC5 receptor |
| MS05 |
TargetsMC1 receptor |
| afamelanotide |
TargetsMC1 receptor; MC3 receptor; MC4 receptor; MC5 receptor |
| [125I]NDP-MSH |
TargetsMC1 receptor; MC3 receptor; MC4 receptor; MC5 receptor |
| ACTH-(1-24) |
TargetsMC2 receptor |
| ACTH {Sp: Mouse, Rat} |
TargetsMC1 receptor; MC2 receptor; MC3 receptor; MC4 receptor; MC5 receptor |
| ACTH-(11-24) |
TargetsMC2 receptor |
| ACTH {Sp: Human} |
TargetsMC1 receptor; MC2 receptor; MC3 receptor; MC4 receptor; MC5 receptor |
| [125I]ACTH-(1-24) |
TargetsMC2 receptor |
| seractide acetate | |
| Pomc (141-162) |
TargetsGPR68 |