GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: compound F8 [PMID: 37145039]
Compound class:
Synthetic organic
Comment: This compound is selectively metabolised to the fluorescent product (4-OH F8) by cytochrome P450 3A4 (CYP3A4) [1]. It is not converted by other P450 isozymes, and its metabolism is inhibited by small molecules that are active against CYP3A/CYP3A4 but not other CYPs. F8 is proposed as a molecular tool for monitoring CYP3A4 activities in living cells and tissue preparations.
|
|
|||||||||||||||||||||||||||||||||||
Classification  |
|
| Compound class | Synthetic organic |
| Is prodrug? | Yes |
| Active form | CYP3A4 fluorescent probe 4-OH F8 |
IUPAC Name  |
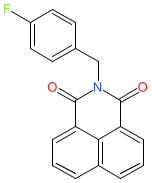
| 2-[(4-fluorophenyl)methyl]benzo[de]isoquinoline-1,3-dione |
Synonyms  |
| compound F8 [PMID: 37145039] |
Database Links  |
|
| BindingDB Ligand | 50070431 |
| ChEMBL Ligand | CHEMBL3408793 |
| GtoPdb PubChem SID | 483123207 |
| PubChem CID | 10614509 |
| Search Google for chemical match using the InChIKey | JVHUQBLLHSIOMR-UHFFFAOYSA-N |
| Search Google for chemicals with the same backbone | JVHUQBLLHSIOMR |
| UniChem Compound Search for chemical match using the InChIKey | JVHUQBLLHSIOMR-UHFFFAOYSA-N |
| UniChem Connectivity Search for chemical match using the InChIKey | JVHUQBLLHSIOMR-UHFFFAOYSA-N |
Product suppliers
View disclaimer
Sponsored products disclaimer
Product supplier links are provided as a service to assist in identifying commercial suppliers of reagents that are mentioned on the IUPHAR/BPS Guide to PHARMACOLOGY database website, and do not imply their endorsement by NC-IUPHAR.
Links are provided in return for sponsorship, used to fund improvements to this database. The sponsorship account is managed and audited by the University of Edinburgh, a charitable body registered in Scotland, SC005336. If you are interested in sponsoring the database, please contact us.
✖
Sponsored products disclaimer
Product supplier links are provided as a service to assist in identifying commercial suppliers of reagents that are mentioned on the IUPHAR/BPS Guide to PHARMACOLOGY database website, and do not imply their endorsement by NC-IUPHAR.
Links are provided in return for sponsorship, used to fund improvements to this database. The sponsorship account is managed and audited by the University of Edinburgh, a charitable body registered in Scotland, SC005336. If you are interested in sponsoring the database, please contact us.
✖|
hCYP3A4 Fluorogenic substrate 1 (links to external site)
Cat. No. HY-155002 |