GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
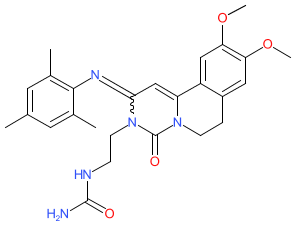
Synonyms: Ohtuvayre® | RPL554 | VMX 554 | VMX-554 | VMX554
ensifentrine is an approved drug (FDA (2024))
Compound class:
Synthetic organic
Comment: Ensifentrine (RPL-554) is an inhibitor of phosphodiesterases 3 and 4 (PDE3 and PDE4) [3]. It has bronchodilatory action that is mediated by inhibition of PDE3 in airway smooth muscle [9,11] that is associated with short-term improvement in lung function [5]. Ensifentrine was developed for therapeutic potential in asthma and chronic obstructive pulmonary disease (COPD) [6-7]. It also also has anti-inflammatory effects in vitro (e.g. reducing TNFα release from human monocytes) and in vivo, likely through inhibtion of PDE4 expressed by inflammatory cells in the lungs [10]. Ensifentrine is active when delivered orally or by inhalation (nebulised) [2].
COVID-19: A pilot study of ensifentrine in hospitalised COVID-19 patients was initiated in mid-late 2020, but by 2024 no results had been published. FDA approval of ensifentrine in 2024 made this the first COPD inhalation drug with a new mechanism of action to reach the clinic in more than 20 years. Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Anzueto A, Barjaktarevic IZ, Siler TM, Rheault T, Bengtsson T, Rickard K, Sciurba F. (2023)
Ensifentrine, a Novel Phosphodiesterase 3 and 4 Inhibitor for the Treatment of Chronic Obstructive Pulmonary Disease: Randomized, Double-Blind, Placebo-controlled, Multicenter Phase III Trials (the ENHANCE Trials). Am J Respir Crit Care Med, 208 (4): 406-416. [PMID:37364283] |
|
2. Bjermer L, Abbott-Banner K, Newman K. (2019)
Efficacy and safety of a first-in-class inhaled PDE3/4 inhibitor (ensifentrine) vs salbutamol in asthma. Pulm Pharmacol Ther, 58: 101814. [PMID:31202957] |
|
3. Boswell-Smith V, Spina D, Oxford AW, Comer MB, Seeds EA, Page CP. (2006)
The pharmacology of two novel long-acting phosphodiesterase 3/4 inhibitors, RPL554 [9,10-dimethoxy-2(2,4,6-trimethylphenylimino)-3-(n-carbamoyl-2-aminoethyl)-3,4,6,7-tetrahydro-2H-pyrimido[6,1-a]isoquinolin-4-one] and RPL565 [6,7-dihydro-2-(2,6-diisopropylphenoxy)-9,10-dimethoxy-4H-pyrimido[6,1-a]isoquinolin-4-one]. J Pharmacol Exp Ther, 318 (2): 840-8. [PMID:16682455] |
|
4. Calzetta L, Pistocchini E, Chetta A, Rogliani P, Cazzola M. (2023)
Experimental drugs in clinical trials for COPD: artificial intelligence via machine learning approach to predict the successful advance from early-stage development to approval. Expert Opin Investig Drugs, 32 (6): 525-536. [PMID:37364225] |
|
5. Cazzola M, Page C. (2018)
An inhaled "bifunctional" dual PDE3/4 inhibitor provides additional short-term improvements in lung function compared to existing classes of bronchodilator: implications for future treatment of COPD. Eur Respir J, 52 (5). DOI: 10.1183/13993003.01675-2018 [PMID:30385603] |
|
6. Cazzola M, Page C, Calzetta L, Matera MG. (2018)
Ensifentrine (RPL554): an inhaled 'bifunctional' dual PDE3/4 inhibitor for the treatment of asthma and chronic obstructive pulmonary disease. Pharm Pat Anal, 7 (6): 249-257. [PMID:30657422] |
|
7. Donohue JF, Rheault T, MacDonald-Berko M, Bengtsson T, Rickard K. (2023)
Ensifentrine as a Novel, Inhaled Treatment for Patients with COPD. Int J Chron Obstruct Pulmon Dis, 18: 1611-1622. [PMID:37533771] |
|
8. Keam SJ. (2024)
Ensifentrine: First Approval. Drugs, 84 (9): 1157-1163. [PMID:39196510] |
|
9. Singh D, Abbott-Banner K, Bengtsson T, Newman K. (2018)
The short-term bronchodilator effects of the dual phosphodiesterase 3 and 4 inhibitor RPL554 in COPD. Eur Respir J, 52 (5). DOI: 10.1183/13993003.01074-2018 [PMID:30166326] |
|
10. Turner MJ, Dauletbaev N, Lands LC, Hanrahan JW. (2020)
The Phosphodiesterase Inhibitor Ensifentrine Reduces Production of Proinflammatory Mediators in Well Differentiated Bronchial Epithelial Cells by Inhibiting PDE4. J Pharmacol Exp Ther, 375 (3): 414-429. [PMID:33012706] |
|
11. Venkatasamy R, Spina D. (2016)
Novel relaxant effects of RPL554 on guinea pig tracheal smooth muscle contractility. Br J Pharmacol, 173 (15): 2335-51. [PMID:27174172] |
|
12. Watz H, Rickard K, Rheault T, Bengtsson T, Singh D. (2020)
Symptom Improvement Following Treatment with the Inhaled Dual Phosphodiesterase 3 and 4 Inhibitor Ensifentrine in Patients with Moderate to Severe COPD - A Detailed Analysis. Int J Chron Obstruct Pulmon Dis, 15: 2199-2206. [PMID:32982212] |