GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: compound 4b [PMID: 28124907] | GS-5734 | GS5734 | Veklury®
remdesivir is an approved drug (FDA & EMA (2020))
Compound class:
Synthetic organic
Comment: Remdesivir (GS-5734) is a nucleotide analogue drug that was developed by Gilead Sciences for antiviral potential, in particular for anti-Ebola and anti-Marburg virus activity [7]. It acts is an inhibitor of the viral RNA-dependent RNA polymerase (RdRP) that functions as a chain terminator during viral RNA synthesis. Remdesivir is a prodrug whose major active metabolite is GS-441524. Its 1'-CN group and C-linked nucleobase ensure optimal anti-Ebola potency and selectivity against host polymerase enzymes. Remdesivir has broad-spectrum activity against several viral families such as filoviruses, paramyxoviruses, and coronaviruses, and including the SARS and MERS coronaviruses (CoVs), endemic circulating human CoVs, and against SARS-CoV-2 [1-2,6]. The first patient to test positive for SARS-CoV-2 in the US was treated with i.v. remdesivir (using compassionate use of an investigational therapy prescribing) and his clinical condition improved significantly within the next 24h without observation of adverse effects [4].
|
|
|||||||||||||||||||||||||||||||||||
Classification  |
|
| Compound class | Synthetic organic |
| Approved drug? | Yes. EU EMA (2020) | US FDA (2020) |
| Is prodrug? | Yes |
| Active form | GS-441524 |
IUPAC Name  |
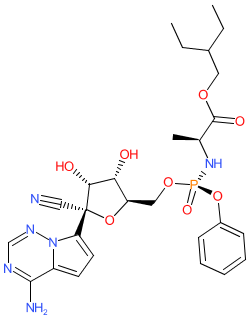
| 2-ethylbutyl (2S)-2-[[[(2R,3S,4R,5R)-5-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-5-cyano-3,4-dihydroxyoxolan-2-yl]methoxy-phenoxyphosphoryl]amino]propanoate |
International Nonproprietary Names  |
|
| INN number | INN |
| 10478 | remdesivir |
Synonyms  |
| compound 4b [PMID: 28124907] | GS-5734 | GS5734 | Veklury® |
Database Links  |
|
| Specialist databases | |
Reactome Drug

|
R-ALL-9680278 |
Reactome Reaction

|
R-HSA-9687384, R-HSA-9687388, R-HSA-9680262, R-HSA-9694665, R-HSA-9694541, R-HSA-9694419 |
| Other databases | |
| CAS Registry No. | 1809249-37-3 (source: WHO INN record) |
| ChEMBL Ligand | CHEMBL4065616 |
| DrugCentral Ligand | 5376 |
| GtoPdb PubChem SID | 404859162 |
| PubChem CID | 121304016 |
| Search Google for chemical match using the InChIKey | RWWYLEGWBNMMLJ-YSOARWBDSA-N |
| Search Google for chemicals with the same backbone | RWWYLEGWBNMMLJ |
| Search PubMed clinical trials | remdesivir |
| Search PubMed titles | remdesivir |
| Search PubMed titles/abstracts | remdesivir |
| UniChem Compound Search for chemical match using the InChIKey | RWWYLEGWBNMMLJ-YSOARWBDSA-N |
| UniChem Connectivity Search for chemical match using the InChIKey | RWWYLEGWBNMMLJ-YSOARWBDSA-N |
Product suppliers
View disclaimer
Sponsored products disclaimer
Product supplier links are provided as a service to assist in identifying commercial suppliers of reagents that are mentioned on the IUPHAR/BPS Guide to PHARMACOLOGY database website, and do not imply their endorsement by NC-IUPHAR.
Links are provided in return for sponsorship, used to fund improvements to this database. The sponsorship account is managed and audited by the University of Edinburgh, a charitable body registered in Scotland, SC005336. If you are interested in sponsoring the database, please contact us.
✖
Sponsored products disclaimer
Product supplier links are provided as a service to assist in identifying commercial suppliers of reagents that are mentioned on the IUPHAR/BPS Guide to PHARMACOLOGY database website, and do not imply their endorsement by NC-IUPHAR.
Links are provided in return for sponsorship, used to fund improvements to this database. The sponsorship account is managed and audited by the University of Edinburgh, a charitable body registered in Scotland, SC005336. If you are interested in sponsoring the database, please contact us.
✖|
Remdesivir (links to external site)
Cat. No. HY-104077 |