|
Synonyms: compound 4b [PMID: 28124907] | GS-5734 | GS5734 | Veklury®
remdesivir is an approved drug (FDA & EMA (2020))
Compound class:
Synthetic organic
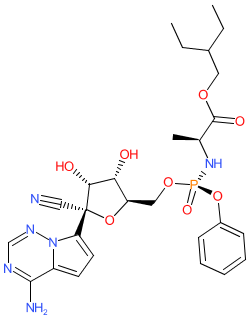
Comment: Remdesivir (GS-5734) is a nucleotide analogue drug that was developed by Gilead Sciences for antiviral potential, in particular for anti-Ebola and anti-Marburg virus activity [7]. It acts is an inhibitor of the viral RNA-dependent RNA polymerase (RdRP) that functions as a chain terminator during viral RNA synthesis. Remdesivir is a prodrug whose major active metabolite is GS-441524. Its 1'-CN group and C-linked nucleobase ensure optimal anti-Ebola potency and selectivity against host polymerase enzymes. Remdesivir has broad-spectrum activity against several viral families such as filoviruses, paramyxoviruses, and coronaviruses, and including the SARS and MERS coronaviruses (CoVs), endemic circulating human CoVs, and against SARS-CoV-2 [1-2,6]. The first patient to test positive for SARS-CoV-2 in the US was treated with i.v. remdesivir (using compassionate use of an investigational therapy prescribing) and his clinical condition improved significantly within the next 24h without observation of adverse effects [4].
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Remdesivir (GS-5734) must be administered by intravenous (i.v.) infusion. Remdesivir progressed to Phase 2 evaluation in Ebola infected patients. It is to be evaluated for efficacy in the treatment of the novel coronavirus (SARS-CoV-2) infection COVID-19, with 4 Phase 3 trials registered on ClinicalTrials.gov as of early March 2020. Grein et al. published results from short-term compassionate-use remdesivir in hospitalised COVID-19 patients (receiving invasive ventilation or ECMO) in the NEJM in April 2020 [3]. Whilst this study indicated some clinical benefit, and detected no additional safety signals, it was not powered to conclusively determine clinical efficacy. The study did not measure the effect of remdesivir on viral load, and it was not placebo-controlled. In some cases remdesivir seems to shorten the time to recovery. Despite limited data on efficacy, the FDA granted full approval in October 2020, for the treatment of hospitalised COVID-19 patients [5]. This was quite unexpected because the FDA based their decision on data from a set of studies that were not suitably designed or controlled, and gave mixed results. It remains the case that larger controlled trials are needed. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT04252664 | Mild/Moderate 2019-nCoV Remdesivir RCT | Phase 3 Interventional | Capital Medical University | ||
| NCT04257656 | Severe 2019-nCoV Remdesivir RCT | Phase 3 Interventional | Capital Medical University | ||
| NCT02818582 | GS-5734 to Assess the Antiviral Activity, Longer-Term Clearance of Ebola Virus, and Safety in Male Ebola Survivors With Evidence of Ebola Virus Persistence in Semen | Phase 2 Interventional | National Institutes of Health Clinical Center (CC) | ||