GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
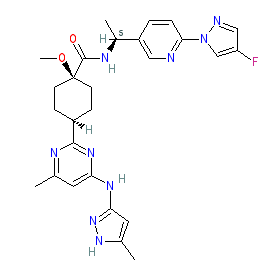
Synonyms: BLU-667 | BLU667 | Gavreto®
pralsetinib is an approved drug (FDA (2020), EMA (2021))
Compound class:
Synthetic organic
Comment: Pralsetinib (BLU-667) is a second-generation selective RET kinase inhibitor that is being developed by Blueprint Medicines [2]. It is Compound 130 in patent US20170121312A1 and our structure was drawn from the image therein [1]. Selective RET inhibitors have oncology potential for the treatment of solid tumours with oncogenic RET alterations (e.g. constitutively activating RET point mutations and RET gene rearrangements). They are expected to offer more effective anti-tumour efficacy in RET-driven tumours than the currently available multi-kinase inhibitors (such as cabozantinib and vandetanib) that have limited anti-RET activity, and which have exhibited poor therapeutic responses in this defined patient group.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Brubaker JD, Kim JL, Wilson KJ, Wilson D, DiPietro LV. (2017)
Inhibitors of ret. Patent number: US20170121312A1. Assignee: Blueprint Medicines Corp. Priority date: 02/11/2015. Publication date: 04/05/2017. |
|
2. Subbiah V, Gainor JF, Rahal R, Brubaker JD, Kim JL, Maynard M, Hu W, Cao Q, Sheets MP, Wilson D et al.. (2018)
Precision Targeted Therapy with BLU-667 for RET-Driven Cancers. Cancer Discov, 8 (7): 836-849. [PMID:29657135] |