GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
Overview
The voltage-gated ion channels and their structural relatives comprise a superfamily encoded by at least 143 genes in the human genome and are therefore one of the largest superfamilies of signal transduction proteins, following the G protein-coupled receptors and the protein kinases in number [1]. In addition to their prominence in signal transduction, these ion channels are also among the most common drug targets. As for other large protein superfamilies, understanding the molecular relationships among family members, developing a unified, rational nomenclature for the ion channel families and subfamilies, and assigning physiological functions and pharmacological significance to each family member has been an important challenge. Some of the ion channels placed under the 'Voltage-gated' umbrella are not in fact gated by voltage, but for the reasons mentioned above it is useful to consider them within this superfamily. The inwardly rectifying potassium channels, two-pore domain potassium channels (K2P), ryanodine receptors (RyR) and transient receptor potential channels (TRP) are those that are NOT voltage-gated.
Subfamilies
|
Families that contain targets of relevance to immunopharmacology are highlighted in blue |
|
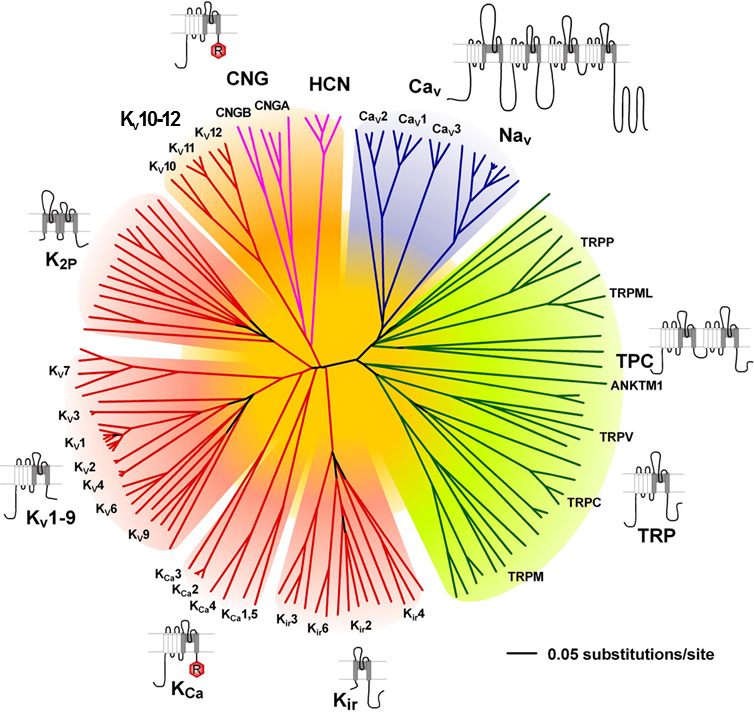
|
|
How to cite this family page
Database page citation:
Voltage-gated ion channels. Accessed on 04/03/2026. IUPHAR/BPS Guide to PHARMACOLOGY, http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=696.
Concise Guide to PHARMACOLOGY citation:
Alexander SPH, Mathie A, Peters JA, Veale EL, Striessnig J, Kelly E, Armstrong JF, Faccenda E, Harding SD, Pawson AJ, Sharman JL, Southan C, Davies JA; CGTP Collaborators. (2019) The Concise Guide to PHARMACOLOGY 2019/20: Ion channels. Br J Pharmacol. 176 Issue S1: S142-228.








The voltage-dependent anion channels (VDACs) plays a key role in regulating metabolic and energetic flux across the outer mitochondrial membrane. It is involved in the transport of ATP, ADP, pyruvate, malate, and other metabolites, and thus communicates extensively with enzymes from metabolic pathways. They are a class of porin ion channel located on the outer mitochondrial membrane. VDAC1, VDAC2 and VDAC3 are involved in the regulation of apoptosis, cell metabolism, mitochondrial apoptosis, and spermatogenesis [3-4].
The calcium homeostasis modulator (CALHM) ion channels are apparently voltage- and extracellular Ca2+-gated, and constitute a novel ion channel family that is widely expressed in the brain and taste buds throughout vertebrates and in sensory neurons and body wall muscles in C. elegans. In humans, the CALHM family encompasses six paralogs, some of which function as non-selective channels that are permeable to large substances such as ATP. CALHM channels are thought to play important roles in neuronal excitability, neurotransmission of tastes, and muscle cell function. The voltage- and extracellular Ca2+-dependent gating mechanisms, structural features that define the gate and ion permeation pathway and additional physiological roles, remain to be discovered [2].