GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
Contents:
- Gene and Protein Information
- Previous and Unofficial Names
- Database Links
- Selected 3D Structures
- Associated Proteins
- Natural/Endogenous Ligands
- Rank order lists
- Agonists
- Antagonists
- Transduction Mechanisms
- Tissue Distribution
- Expression Datasets
- Functional Assays
- Physiological Functions
- Physiological Consequences of Altering Gene Expression
- Phenotypes, Alleles and Disease Models
- Biologically Significant Variants
- General Comments
- References
- Contributors
- How to cite this page
Gene and Protein Information  |
||||||
| class A G protein-coupled receptor | ||||||
| Species | TM | AA | Chromosomal Location | Gene Symbol | Gene Name | Reference |
| Human | 7 | 408 | 8p11.23 | ADRB3 | adrenoceptor beta 3 | 29,53 |
| Mouse | 7 | 400 | 8 15.94 cM | Adrb3 | adrenergic receptor, beta 3 | 64 |
| Rat | 7 | 400 | 16q12.3 | Adrb3 | adrenoceptor beta 3 | 36,61 |
Previous and Unofficial Names  |
| atypical β-adrenoceptor | ADRB | beta-3 adrenoreceptor | Adrb-3 | beta 3-AR | beta3-adrenergic receptor | adrenergic receptor |
Database Links  |
|
| Specialist databases | |
| GPCRdb | adrb3_human (Hs), adrb3_mouse (Mm), adrb3_rat (Rn) |
| Other databases | |
| Alphafold | P13945 (Hs), P25962 (Mm), P26255 (Rn) |
| ChEMBL Target | CHEMBL246 (Hs), CHEMBL4030 (Mm), CHEMBL4031 (Rn) |
| DrugBank Target | P13945 (Hs) |
| Ensembl Gene | ENSG00000188778 (Hs), ENSMUSG00000031489 (Mm), ENSRNOG00000012674 (Rn) |
| Entrez Gene | 155 (Hs), 11556 (Mm), 25645 (Rn) |
| Human Protein Atlas | ENSG00000188778 (Hs) |
| KEGG Gene | hsa:155 (Hs), mmu:11556 (Mm), rno:25645 (Rn) |
| OMIM | 109691 (Hs) |
| Pharos | P13945 (Hs) |
| RefSeq Nucleotide | NM_000025 (Hs), NM_013462 (Mm), NM_013108 (Rn) |
| RefSeq Protein | NP_000016 (Hs), NP_038490 (Mm), NP_037240 (Rn) |
| UniProtKB | P13945 (Hs), P25962 (Mm), P26255 (Rn) |
| Wikipedia | ADRB3 (Hs) |
Selected 3D Structures  |
|||||||||||||
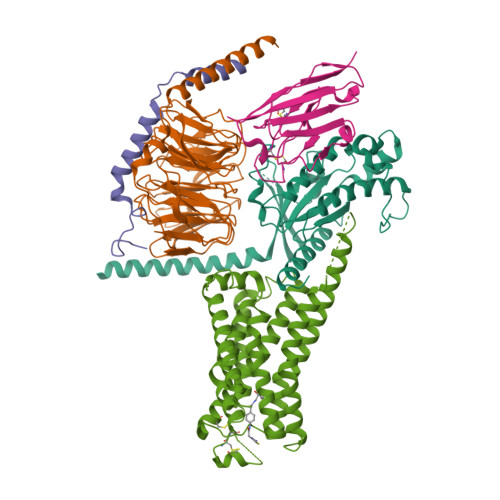
|
|
||||||||||||
Associated Proteins  |
|||||||||||||||
|
|||||||||||||||
Natural/Endogenous Ligands  |
| (-)-adrenaline |
| (-)-noradrenaline |
| Potency order of endogenous ligands (Human) |
| (-)-noradrenaline = (-)-adrenaline |
Download all structure-activity data for this target as a CSV file 
| Agonists | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| View species-specific agonist tables | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Agonist Comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| The species orthologs of the human β-ARs found in the rat, mouse, and cow have significantly different agonist pharmacology that has proved problematic for drug development. For example, BRL37344 is a potent agonist at rodent β3-AR but a weak partial agonist at the human β3-AR [2] and carazolol has a much higher affinity for the bovine receptor [69], whereas [125I]CYP has lower affinity [85]. Carazolol is a potent high efficacy partial agonist at β3-AR but is also a potent partial agonist at β1-AR and a highly potent antagonist at β2-AR [5]. Agonist SB251023 has an pEC50 of 6.9 for the splice variant of the mouse β3 receptor, β3b [43]. For the agonist CL316243, a cAMP accumulation assay measured pEC50 values of 9.94 and 9.97 for the β3a and β3b splice variants respectively [78]. Although the human β3-AR has an intron no splice variants have been observed. The β3-AR has a more important physiological role in rodents than in humans. Since the β3-AR largely lacks sites required for receptor phosphorylation it is less susceptible to desensitisation than other β-AR subtypes. However, desensitisation can occur associated with mRNA and protein down regulation of receptor and post receptor signalling proteins and there is also evidence for cell specific events [66]. Ligands acting at β3-AR can also display ligand-directed signalling [76-77]. Vibegron, solabegron and mirabegron are selective β3-AR agonists that potently activate human β3-AR [45]. Clinical uses: β3-AR agonists are used to treat overactive bladder syndrome. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Antagonists | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| View species-specific antagonist tables | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Antagonist Comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Many of the compounds listed as antagonists at the β3-AR can behave in some systems as agonists at the β3-AR. These include L748337, SR59230A, carvedilol, nadolol, tertatolol, pindolol and propranolol as well as compounds that exhibit similar behaviour at other β-AR subtypes, notably CGP12177, pindolol, cyanopindolol, labetalol and alprenolol [3,5,10-11,27,62,89]. The approved drug propranolol is primarily a β1-/β2-AR antagonist with lower affinity for β3-AR. SR59230A that is often described as a selective antagonist at β3-AR displays little selectivity and has similar potency at all 3 subtypes (in fact higher affinity at β2-AR) [5]- what it does have is reasonable potency at blocking β3-AR. L-748337 is currently the most selective antagonist at the human β3-AR [96]. The human β3-AR also appears to exist in two active conformations (see also β1-AR) with fenoterol stimulating responses via the catecholamine formation whereas alprenolol, SR58230A and CGP12177 utilise the secondary conformation [3]. Clinical uses: β3-AR antagonists are not used clinically. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Primary Transduction Mechanisms 
|
|
| Transducer | Effector/Response |
| Gs family | |
| Comments: Agonist stimulated Gs coupling leads to stimulation of adenylyl cyclase (AC) and causes the conversion of ATP into cAMP. This activates protein kinase A (PKA) that in adipocytes leads to phosphorylation and activation of the hormone sensitive lipase and perilipins. Other kinases including ERK1/2, p38MAPK and AMP kinases may also be activated and PKA may be involved. In other tissues such as gut cAMP generation leads to relaxation. | |
| References: 18,22,51,73,82 | |
Secondary Transduction Mechanisms  |
|
| Transducer | Effector/Response |
| Gi/Go family | Guanylate cyclase stimulation |
| Comments: β3-ARs can also couple to Gi to inhibit adenylyl cyclase activity and reduce cAMP levels in some systems. βγ subunits from Gi are also likely involved in ERK1/2 phosphorylation following β3-AR activation. β3-ARs can also stimulate nitric oxide production through the activation of endothelial nitric oxide synthase. Nitric oxide activates guanylyl cyclase and increases cGMP levels. | |
| References: 22,30,37,54,73,93,97 | |
Tissue Distribution 
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
Expression Datasets  |
|
|
Functional Assays 
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
Physiological Functions 
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
Physiological Consequences of Altering Gene Expression 
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
Phenotypes, Alleles and Disease Models 
|
Mouse data from MGI | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Biologically Significant Variants 
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
| General Comments |
| For a review on the β-adrenoceptor polymorphisms see reference [52], however it should be noted that many of the reported polymorphisms associated with obesity and diabetes have not been consistently reproduced. |
References
1. Aparici M, Gómez-Angelats M, Vilella D, Otal R, Carcasona C, Viñals M, Ramos I, Gavaldà A, De Alba J, Gras J et al.. (2012) Pharmacological characterization of abediterol, a novel inhaled β(2)-adrenoceptor agonist with long duration of action and a favorable safety profile in preclinical models. J Pharmacol Exp Ther, 342 (2): 497-509. [PMID:22588259]
2. Arch JR. (2011) Challenges in β(3)-Adrenoceptor Agonist Drug Development. Ther Adv Endocrinol Metab, 2 (2): 59-64. [PMID:23148171]
3. Baker JG. (2005) Evidence for a secondary state of the human beta3-adrenoceptor. Mol Pharmacol, 68 (6): 1645-55. [PMID:16129733]
4. Baker JG. (2005) The selectivity of beta-adrenoceptor antagonists at the human beta1, beta2 and beta3 adrenoceptors. Br J Pharmacol, 144 (3): 317-22. [PMID:15655528]
5. Baker JG. (2010) The selectivity of beta-adrenoceptor agonists at human beta1-, beta2- and beta3-adrenoceptors. Br J Pharmacol, 160 (5): 1048-61. [PMID:20590599]
6. Bardou M, Dousset B, Deneux-Tharaux C, Smadja C, Naline E, Chaput JC, Naveau S, Manara L, Croci T, Advenier C. (1998) In vitro inhibition of human colonic motility with SR 59119A and SR 59104A: evidence of a beta3-adrenoceptor-mediated effect. Eur J Pharmacol, 353 (2-3): 281-7. [PMID:9726658]
7. Baskin AS, Linderman JD, Brychta RJ, McGehee S, Anflick-Chames E, Cero C, Johnson JW, O'Mara AE, Fletcher LA, Leitner BP et al.. (2018) Regulation of Human Adipose Tissue Activation, Gallbladder Size, and Bile Acid Metabolism by a β3-Adrenergic Receptor Agonist. Diabetes, 67 (10): 2113-2125. [PMID:29980535]
8. Berbée JF, Boon MR, Khedoe PP, Bartelt A, Schlein C, Worthmann A, Kooijman S, Hoeke G, Mol IM, John C et al.. (2015) Brown fat activation reduces hypercholesterolaemia and protects from atherosclerosis development. Nat Commun, 6: 6356. [PMID:25754609]
9. Berkowitz DE, Nardone NA, Smiley RM, Price DT, Kreutter DK, Fremeau RT, Schwinn DA. (1995) Distribution of beta 3-adrenoceptor mRNA in human tissues. Eur J Pharmacol, 289: 223-228. [PMID:7621895]
10. Blin N, Camoin L, Maigret B, Strosberg AD. (1993) Structural and conformational features determining selective signal transduction in the beta 3-adrenergic receptor. Mol Pharmacol, 44 (6): 1094-104. [PMID:7903415]
11. Blin N, Nahmias C, Drumare MF, Strosberg AD. (1994) Mediation of most atypical effects by species homologues of the beta 3-adrenoceptor. Br J Pharmacol, 112 (3): 911-9. [PMID:7921620]
12. Breit A, Lagacé M, Bouvier M. (2004) Hetero-oligomerization between beta2- and beta3-adrenergic receptors generates a beta-adrenergic signaling unit with distinct functional properties. J Biol Chem, 279 (27): 28756-65. [PMID:15123695]
13. Brixius K, Bloch W, Pott C, Napp A, Krahwinkel A, Ziskoven C, Koriller M, Mehlhorn U, Hescheler J, Fleischmann B et al.. (2004) Mechanisms of beta 3-adrenoceptor-induced eNOS activation in right atrial and left ventricular human myocardium. Br J Pharmacol, 143 (8): 1014-22. [PMID:15466444]
14. Brucker BM, King J, Mudd Jr PN, McHale K. (2022) Selectivity and Maximum Response of Vibegron and Mirabegron for β3-Adrenergic Receptors. Curr Ther Res Clin Exp, 96: 100674. [PMID:35693456]
15. Candelore MR, Deng L, Tota L, Guan XM, Amend A, Liu Y, Newbold R, Cascieri MA, Weber AE. (1999) Potent and selective human beta(3)-adrenergic receptor antagonists. J Pharmacol Exp Ther, 290 (2): 649-55. [PMID:10411574]
16. Cao W, Luttrell LM, Medvedev AV, Pierce KL, Daniel KW, Dixon TM, Lefkowitz RJ, Collins S. (2000) Direct binding of activated c-Src to the beta 3-adrenergic receptor is required for MAP kinase activation. J Biol Chem, 275 (49): 38131-4. [PMID:11013230]
17. Cero C, Lea HJ, Zhu KY, Shamsi F, Tseng YH, Cypess AM. (2021) β3-Adrenergic receptors regulate human brown/beige adipocyte lipolysis and thermogenesis. JCI Insight, 6 (11). [PMID:34100382]
18. Chernogubova E, Cannon B, Bengtsson T. (2004) Norepinephrine increases glucose transport in brown adipocytes via beta3-adrenoceptors through a cAMP, PKA, and PI3-kinase-dependent pathway stimulating conventional and novel PKCs. Endocrinology, 145: 269-280. [PMID:14551227]
19. Chernogubova E, Hutchinson DS, Nedergaard J, Bengtsson T. (2005) Alpha1- and beta1-adrenoceptor signaling fully compensates for beta3-adrenoceptor deficiency in brown adipocyte norepinephrine-stimulated glucose uptake. Endocrinology, 146 (5): 2271-84. [PMID:15665039]
20. Christiansen C, Poulsen P, Beck-Nielsen H. (1999) The Trp64Arg mutation of the adrenergic beta-3 receptor gene impairs insulin secretion: a twin study. Diabet Med, 16 (10): 835-40. [PMID:10547210]
21. Clement K, Vaisse C, Manning BS, Basdevant A, Guy-Grand B, Ruiz J, Silver KD, Shuldiner AR, Froguel P, Strosberg AD. (1995) Genetic variation in the beta 3-adrenergic receptor and an increased capacity to gain weight in patients with morbid obesity. N Engl J Med, 333: 352-354. [PMID:7609752]
22. Collins S. (2011) β-Adrenoceptor Signaling Networks in Adipocytes for Recruiting Stored Fat and Energy Expenditure. Front Endocrinol (Lausanne), 2: 102. [PMID:22654837]
23. De Ponti F, Gibelli G, Croci T, Arcidiaco M, Crema F, Manara L. (1996) Functional evidence of atypical beta 3-adrenoceptors in the human colon using the beta 3-selective adrenoceptor antagonist, SR 59230A. Br J Pharmacol, 117 (7): 1374-6. [PMID:8730727]
24. Dehvari N, Sato M, Bokhari MH, Kalinovich A, Ham S, Gao J, Nguyen HTM, Whiting L, Mukaida S, Merlin J et al.. (2020) The metabolic effects of mirabegron are mediated primarily by β3 -adrenoceptors. Pharmacol Res Perspect, 8 (5): e00643. [PMID:32813332]
25. Dessy C, Moniotte S, Ghisdal P, Havaux X, Noirhomme P, Balligand JL. (2004) Endothelial beta3-adrenoceptors mediate vasorelaxation of human coronary microarteries through nitric oxide and endothelium-dependent hyperpolarization. Circulation, 110 (8): 948-54. [PMID:15302798]
26. Di Salvo J, Nagabukuro H, Wickham LA, Abbadie C, DeMartino JA, Fitzmaurice A, Gichuru L, Kulick A, Donnelly MJ, Jochnowitz N et al.. (2017) Pharmacological Characterization of a Novel Beta 3 Adrenergic Agonist, Vibegron: Evaluation of Antimuscarinic Receptor Selectivity for Combination Therapy for Overactive Bladder. J Pharmacol Exp Ther, 360 (2): 346-355. [PMID:27965369]
27. Dolan JA, Muenkel HA, Burns MG, Pellegrino SM, Fraser CM, Pietri F, Strosberg AD, Largis EE, Dutia MD, Bloom JD et al.. (1994) Beta-3 adrenoceptor selectivity of the dioxolane dicarboxylate phenethanolamines. J Pharmacol Exp Ther, 269 (3): 1000-6. [PMID:7912272]
28. Edmondson SD, Zhu C, Kar NF, Di Salvo J, Nagabukuro H, Sacre-Salem B, Dingley K, Berger R, Goble SD, Morriello G et al.. (2016) Discovery of Vibegron: A Potent and Selective β3 Adrenergic Receptor Agonist for the Treatment of Overactive Bladder. J Med Chem, 59 (2): 609-23. [PMID:26709102]
29. Emorine LJ, Marullo S, Briend-Sutren MM, Patey G, Tate K, Delavier-Klutchko C, Strosberg AD. (1989) Molecular characterization of the human beta 3-adrenergic receptor. Science, 245 (4922): 1118-21. [PMID:2570461]
30. Feng MG, Prieto MC, Navar LG. (2012) Nebivolol-induced vasodilation of renal afferent arterioles involves β3-adrenergic receptor and nitric oxide synthase activation. Am J Physiol Renal Physiol, 303 (5): F775-82. [PMID:22674024]
31. Festa A, Krugluger W, Shnawa N, Hopmeier P, Haffner SM, Schernthaner G. (1999) Trp64Arg polymorphism of the beta3-adrenergic receptor gene in pregnancy: association with mild gestational diabetes mellitus. J Clin Endocrinol Metab, 84 (5): 1695-9. [PMID:10323402]
32. Fletcher DS, Candelore MR, Grujic D, Lowell BB, Luell S, Susulic VS, Macintyre DE. (1998) Beta-3 adrenergic receptor agonists cause an increase in gastrointestinal transit time in wild-type mice, but not in mice lacking the beta-3 adrenergic receptor. J Pharmacol Exp Ther, 287 (2): 720-4. [PMID:9808702]
33. Frazier EP, Michel-Reher MB, van Loenen P, Sand C, Schneider T, Peters SL, Michel MC. (2011) Lack of evidence that nebivolol is a β₃-adrenoceptor agonist. Eur J Pharmacol, 654 (1): 86-91. [PMID:21172342]
34. Gauthier C, Tavernier G, Charpentier F, Langin D, Le Marec H. (1996) Functional beta3-adrenoceptor in the human heart. J Clin Invest, 98 (2): 556-62. [PMID:8755668]
35. Germack R, Starzec AB, Vassy R, Perret GY. (1997) Beta-adrenoceptor subtype expression and function in rat white adipocytes. Br J Pharmacol, 120 (2): 201-10. [PMID:9117110]
36. Granneman JG, Lahners KN, Chaudhry A. (1991) Molecular cloning and expression of the rat beta 3-adrenergic receptor. Mol Pharmacol, 40: 895-899. [PMID:1684635]
37. Hadi T, Barrichon M, Mourtialon P, Wendremaire M, Garrido C, Sagot P, Bardou M, Lirussi F. (2013) Biphasic Erk1/2 activation sequentially involving Gs and Gi signaling is required in beta3-adrenergic receptor-induced primary smooth muscle cell proliferation. Biochim Biophys Acta, 1833 (5): 1041-51. [PMID:23388888]
38. Hatanaka T, Ukai M, Watanabe M, Someya A, Ohtake A, Suzuki M, Ueshima K, Sato S, Sasamata M. (2013) In vitro and in vivo pharmacological profile of the selective β3-adrenoceptor agonist mirabegron in rats. Naunyn Schmiedebergs Arch Pharmacol, 386 (3): 247-53. [PMID:23239087]
39. Hermida N, Michel L, Esfahani H, Dubois-Deruy E, Hammond J, Bouzin C, Markl A, Colin H, Steenbergen AV, De Meester C et al.. (2018) Cardiac myocyte β3-adrenergic receptors prevent myocardial fibrosis by modulating oxidant stress-dependent paracrine signaling. Eur Heart J, 39 (10): 888-898. [PMID:29106524]
40. Hoffmann C, Leitz MR, Oberdorf-Maass S, Lohse MJ, Klotz KN. (2004) Comparative pharmacology of human beta-adrenergic receptor subtypes--characterization of stably transfected receptors in CHO cells. Naunyn Schmiedebergs Arch Pharmacol, 369 (2): 151-9. [PMID:14730417]
41. Honda K, Yamaguchi O, Nomiya M, Shishido K, Ishibashi K, Takahashi N, Aikawa K. (2014) Association between polymorphism of beta3-adrenoceptor gene and overactive bladder. Neurourol Urodyn, 33 (4): 400-2. [PMID:24038238]
42. Huang Q, Yang TL, Tang BS, Chen X, Huang X, Luo XH, Zhu YS, Chen XP, Hu PC, Chen J et al.. (2013) Two novel functional single nucleotide polymorphisms of ADRB3 are associated with type 2 diabetes in the Chinese population. J Clin Endocrinol Metab, 98 (7): E1272-7. [PMID:23640967]
43. Hutchinson DS, Bengtsson T, Evans BA, Summers RJ. (2002) Mouse beta 3a- and beta 3b-adrenoceptors expressed in Chinese hamster ovary cells display identical pharmacology but utilize distinct signalling pathways. Br J Pharmacol, 135 (8): 1903-14. [PMID:11959793]
44. Hutchinson DS, Chernogubova E, Sato M, Summers RJ, Bengtsson T. (2006) Agonist effects of zinterol at the mouse and human beta(3)-adrenoceptor. Naunyn Schmiedebergs Arch Pharmacol, 373 (2): 158-68. [PMID:16601951]
45. Igawa Y, Michel MC. (2013) Pharmacological profile of β3-adrenoceptor agonists in clinical development for the treatment of overactive bladder syndrome. Naunyn Schmiedebergs Arch Pharmacol, 386 (3): 177-83. [PMID:23263450]
46. Jockers R, Issad T, Zilberfarb V, de Coppet P, Marullo S, Strosberg AD. (1998) Desensitization of the beta-adrenergic response in human brown adipocytes. Endocrinology, 139: 2676-2684. [PMID:9607772]
47. Kannabiran SA, Gosejacob D, Niemann B, Nikolaev VO, Pfeifer A. (2020) Real-time monitoring of cAMP in brown adipocytes reveals differential compartmentation of β1 and β3-adrenoceptor signalling. Mol Metab, 37: 100986. [PMID:32247064]
48. Kimura K, Sasaki N, Asano A, Mizukami J, Kayahashi S, Kawada T, Fushiki T, Morimatsu M, Yoshida T, Saito M. (2000) Mutated human beta3-adrenergic receptor (Trp64Arg) lowers the response to beta3-adrenergic agonists in transfected 3T3-L1 preadipocytes. Horm Metab Res, 32 (3): 91-6. [PMID:10786926]
49. Kohout TA, Takaoka H, McDonald PH, Perry SJ, Mao L, Lefkowitz RJ, Rockman HA. (2001) Augmentation of cardiac contractility mediated by the human beta(3)-adrenergic receptor overexpressed in the hearts of transgenic mice. Circulation, 104 (20): 2485-91. [PMID:11705829]
50. Krief S, Lonnqvist F, Raimbault S, Baude B, Van Spronsen A, Arner P, Strosberg AD, Ricquier D, Emorine LJ. (1993) Tissue distribution of beta 3-adrenergic receptor mRNA in man. J Clin Invest, 91: 344-349. [PMID:8380813]
51. Large V, Arner P, Reynisdottir S, Grober J, Van Harmelen V, Holm C, Langin D. (1998) Hormone-sensitive lipase expression and activity in relation to lipolysis in human fat cells. J Lipid Res, 39: 1688-1695. [PMID:9717730]
52. Leineweber K, Büscher R, Bruck H, Brodde OE. (2004) Beta-adrenoceptor polymorphisms. Naunyn Schmiedebergs Arch Pharmacol, 369 (1): 1-22. [PMID:14647973]
53. Lelias JM, Kaghad M, Rodriguez M, Chalon P, Bonnin J, Dupre I, Delpech B, Bensaid M, LeFur G, Ferrara P et al.. (1993) Molecular cloning of a human beta 3-adrenergic receptor cDNA. FEBS Lett, 324 (2): 127-30. [PMID:8389717]
54. Li F, De Godoy M, Rattan S. (2004) Role of adenylate and guanylate cyclases in beta1-, beta2-, and beta3-adrenoceptor-mediated relaxation of internal anal sphincter smooth muscle. J Pharmacol Exp Ther, 308 (3): 1111-20. [PMID:14711933]
55. Lonnqvist F, Krief S, Strosberg AD, Nyberg S, Emorine LJ, Arner P. (1993) Evidence for a functional beta 3-adrenoceptor in man. Br J Pharmacol, 110: 929-936. [PMID:7905344]
56. Louis SN, Nero TL, Iakovidis D, Jackman GP, Louis WJ. (1999) LK 204-545, a highly selective beta1-adrenoceptor antagonist at human beta-adrenoceptors. Eur J Pharmacol, 367 (2-3): 431-5. [PMID:10079020]
57. Matsushita M, Horinouchi T, Tanaka Y, Tsuru H, Koike K. (2003) Characterization of beta 3-adrenoceptor-mediated relaxation in rat abdominal aorta smooth muscle. Eur J Pharmacol, 482 (1-3): 235-44. [PMID:14660028]
58. Melis MG, Secchi G, Brizzi P, Severino C, Maioli M, Tonolo G. (2002) The Trp64Arg beta3-adrenergic receptor amino acid variant confers increased sensitivity to the pressor effects of noradrenaline in Sardinian subjects. Clin Sci, 103 (4): 397-402. [PMID:12241539]
59. Michel MC, Korstanje C. (2016) β3-Adrenoceptor agonists for overactive bladder syndrome: Role of translational pharmacology in a repositioning clinical drug development project. Pharmacol Ther, 159: 66-82. [PMID:26808167]
60. Molenaar P, Sarsero D, Arch JR, Kelly J, Henson SM, Kaumann AJ. (1997) Effects of (-)-RO363 at human atrial beta-adrenoceptor subtypes, the human cloned beta 3-adrenoceptor and rodent intestinal beta 3-adrenoceptors. Br J Pharmacol, 120 (2): 165-76. [PMID:9117106]
61. Muzzin P, Revelli JP, Kuhne F, Gocayne JD, McCombie WR, Venter JC, Giacobino JP, Fraser CM. (1991) An adipose tissue-specific beta-adrenergic receptor. Molecular cloning and down-regulation in obesity. J Biol Chem, 266 (35): 24053-8. [PMID:1721063]
62. Méjean A, Guillaume JL, Strosberg AD. (1995) Carazolol: a potent, selective beta 3-adrenoceptor agonist. Eur J Pharmacol, 291 (3): 359-66. [PMID:8719421]
63. Nagiri C, Kobayashi K, Tomita A, Kato M, Kobayashi K, Yamashita K, Nishizawa T, Inoue A, Shihoya W, Nureki O. (2021) Cryo-EM structure of the β3-adrenergic receptor reveals the molecular basis of subtype selectivity. Mol Cell, 81 (15): 3205-3215.e5. [PMID:34314699]
64. Nahmias C, Blin N, Elalouf JM, Mattei MG, Strosberg AD, Emorine LJ. (1991) Molecular characterization of the mouse beta 3-adrenergic receptor: relationship with the atypical receptor of adipocytes. EMBO J, 10 (12): 3721-7. [PMID:1718744]
65. Ochoa MC, Marti A, Azcona C, Chueca M, Oyarzábal M, Pelach R, Patiño A, Moreno-Aliaga MJ, Martínez-González MA, Martínez JA et al.. (2004) Gene-gene interaction between PPAR gamma 2 and ADR beta 3 increases obesity risk in children and adolescents. Int J Obes Relat Metab Disord, 28 Suppl 3: S37-41. [PMID:15543217]
66. Okeke K, Angers S, Bouvier M, Michel MC. (2019) Agonist-induced desensitisation of β3 -adrenoceptors: Where, when, and how?. Br J Pharmacol, 176 (14): 2539-2558. [PMID:30809805]
67. Olsen JM, Sato M, Dallner OS, Sandström AL, Pisani DF, Chambard JC, Amri EZ, Hutchinson DS, Bengtsson T. (2014) Glucose uptake in brown fat cells is dependent on mTOR complex 2-promoted GLUT1 translocation. J Cell Biol, 207 (3): 365-74. [PMID:25385184]
68. Oostendorp J, Preitner F, Moffatt J, Jimenez M, Giacobino JP, Molenaar P, Kaumann AJ. (2000) Contribution of beta-adrenoceptor subtypes to relaxation of colon and oesophagus and pacemaker activity of ureter in wildtype and beta(3)-adrenoceptor knockout mice. Br J Pharmacol, 130 (4): 747-58. [PMID:10864880]
69. Piétri-Rouxel F, Lenzen G, Kapoor A, Drumare MF, Archimbault P, Strosberg AD, Manning BS. (1995) Molecular cloning and pharmacological characterization of the bovine beta 3-adrenergic receptor. Eur J Biochem, 230 (1): 350-8. [PMID:7601122]
70. Popp BD, Hutchinson DS, Evans BA, Summers RJ. (2004) Stereoselectivity for interactions of agonists and antagonists at mouse, rat and human beta3-adrenoceptors. Eur J Pharmacol, 484 (2-3): 323-31. [PMID:14744619]
71. Ramis JM, González-Sánchez JL, Proenza AM, Martínez-Larrad MT, Fernández-Pérez C, Palou A, Serrano-Ríos M. (2004) The Arg64 allele of the beta 3-adrenoceptor gene but not the -3826G allele of the uncoupling protein 1 gene is associated with increased leptin levels in the Spanish population. Metab Clin Exp, 53 (11): 1411-6. [PMID:15536594]
72. Roberts SJ, Papaioannou M, Evans BA, Summers RJ. (1997) Functional and molecular evidence for beta 1-, beta 2- and beta 3- adrenoceptors in human colon. Br J Pharmacol, 120 (8): 1527-35. [PMID:9113375]
73. Roberts SJ, Papaioannou M, Evans BA, Summers RJ. (1999) Characterization of beta-adrenoceptor mediated smooth muscle relaxation and the detection of mRNA for beta1-, beta2- and beta3-adrenoceptors in rat ileum. Br J Pharmacol, 127 (4): 949-61. [PMID:10433503]
74. Rouget C, Bardou M, Breuiller-Fouché M, Loustalot C, Qi H, Naline E, Croci T, Cabrol D, Advenier C, Leroy MJ. (2005) Beta3-adrenoceptor is the predominant beta-adrenoceptor subtype in human myometrium and its expression is up-regulated in pregnancy. J Clin Endocrinol Metab, 90 (3): 1644-50. [PMID:15585565]
75. Rouget C, Rekik M, Camparo P, Botto H, Rischmann P, Lluel P, Palea S, Westfall TD. (2014) Modulation of nerve-evoked contractions by β3-adrenoceptor agonism in human and rat isolated urinary bladder. Pharmacol Res, 80: 14-20. [PMID:24378642]
76. Sato M, Horinouchi T, Hutchinson DS, Evans BA, Summers RJ. (2007) Ligand-directed signaling at the beta3-adrenoceptor produced by 3-(2-Ethylphenoxy)-1-[(1,S)-1,2,3,4-tetrahydronapth-1-ylamino]-2S-2-propanol oxalate (SR59230A) relative to receptor agonists. Mol Pharmacol, 72 (5): 1359-68. [PMID:17717109]
77. Sato M, Hutchinson DS, Evans BA, Summers RJ. (2008) The beta3-adrenoceptor agonist 4-[[(Hexylamino)carbonyl]amino]-N-[4-[2-[[(2S)-2-hydroxy-3-(4-hydroxyphenoxy)propyl]amino]ethyl]-phenyl]-benzenesulfonamide (L755507) and antagonist (S)-N-[4-[2-[[3-[3-(acetamidomethyl)phenoxy]-2-hydroxypropyl]amino]-ethyl]phenyl]benzenesulfonamide (L748337) activate different signaling pathways in Chinese hamster ovary-K1 cells stably expressing the human beta3-adrenoceptor. Mol Pharmacol, 74 (5): 1417-28. [PMID:18684840]
78. Sato M, Hutchinson DS, Halls ML, Furness SG, Bengtsson T, Evans BA, Summers RJ. (2012) Interaction with caveolin-1 modulates G protein coupling of mouse β3-adrenoceptor. J Biol Chem, 287 (24): 20674-88. [PMID:22535965]
79. Sato Y, Kurose H, Isogaya M, Nagao T. (1996) Molecular characterization of pharmacological properties of T-0509 for beta-adrenoceptors. Eur J Pharmacol, 315 (3): 363-7. [PMID:8982677]
80. Severi C, Tattoli I, Romano G, Corleto VD, Delle Fave G. (2004) Beta3-adrenoceptors: relaxant function and mRNA detection in smooth muscle cells isolated from the human colon. Can J Physiol Pharmacol, 82 (7): 515-22. [PMID:15389299]
81. Shihara N, Yasuda K, Moritani T, Ue H, Adachi T, Tanaka H, Tsuda K, Seino Y. (1999) The association between Trp64Arg polymorphism of the beta3-adrenergic receptor and autonomic nervous system activity. J Clin Endocrinol Metab, 84 (5): 1623-7. [PMID:10323390]
82. Skeberdis VA, Jurevicius J, Fischmeister R. (1999) b3-adrenergic regulation of cardiac L-type Ca2+ current in human atrial myocytes. Biophys J, 76: A343-A343.
83. Snitker S, Odeleye OE, Hellmer J, Boschmann M, Monroe MB, Shuldiner AR, Ravussin E. (1997) No effect of the Trp64Arg beta 3-adrenoceptor variant on in vivo lipolysis in subcutaneous adipose tissue. Diabetologia, 40: 838-842. [PMID:9243106]
84. Souza-Braga P, Lorena FB, Nascimento BPP, Marcelino CP, Ravache TT, Ricci E, Bernardi MM, Ribeiro MO. (2018) Adrenergic receptor β3 is involved in the memory consolidation process in mice. Braz J Med Biol Res, 51 (10): e7564. [PMID:30088540]
85. Strosberg AD. (1997) Structure and function of the beta 3-adrenergic receptor. Annu Rev Pharmacol Toxicol, 37: 421-50. [PMID:9131260]
86. Susulic VS, Frederich RC, Lawitts J, Tozzo E, Kahn BB, Harper ME, Himms-Hagen J, Flier JS, Lowell BB. (1995) Targeted disruption of the beta 3-adrenergic receptor gene. J Biol Chem, 270 (49): 29483-92. [PMID:7493988]
87. Suzuki T, Otsuka A, Matsumoto R, Furuse H, Ozono S. (2016) The expression of β3-adrenoceptors and their function in the human prostate. Prostate, 76 (2): 163-71. [PMID:26768278]
88. Takasu T, Ukai M, Sato S, Matsui T, Nagase I, Maruyama T, Sasamata M, Miyata K, Uchida H, Yamaguchi O. (2007) Effect of (R)-2-(2-aminothiazol-4-yl)-4'-{2-[(2-hydroxy-2-phenylethyl)amino]ethyl} acetanilide (YM178), a novel selective beta3-adrenoceptor agonist, on bladder function. J Pharmacol Exp Ther, 321 (2): 642-7. [PMID:17293563]
89. Tate KM, Briend-Sutren MM, Emorine LJ, Delavier-Klutchko C, Marullo S, Strosberg AD. (1991) Expression of three human beta-adrenergic-receptor subtypes in transfected Chinese hamster ovary cells. Eur J Biochem, 196 (2): 357-61. [PMID:1848818]
90. Tavernier G, Barbe P, Galitzky J, Berlan M, Caput D, Lafontan M, Langin D. (1996) Expression of beta3-adrenoceptors with low lipolytic action in human subcutaneous white adipocytes. J Lipid Res, 37 (1): 87-97. [PMID:8820105]
91. Tavernier G, Toumaniantz G, Erfanian M, Heymann MF, Laurent K, Langin D, Gauthier C. (2003) beta3-Adrenergic stimulation produces a decrease of cardiac contractility ex vivo in mice overexpressing the human beta3-adrenergic receptor. Cardiovasc Res, 59 (2): 288-96. [PMID:12909312]
92. Tonolo G, Melis MG, Secchi G, Atzeni MM, Angius MF, Carboni A, Ciccarese M, Malavasi A, Maioli M. (1999) Association of Trp64Arg beta 3-adrenergic-receptor gene polymorphism with essential hypertension in the Sardinian population. J Hypertens, 17 (1): 33-8. [PMID:10100091]
93. Trochu JN, Leblais V, Rautureau Y, Bévérelli F, Le Marec H, Berdeaux A, Gauthier C. (1999) Beta 3-adrenoceptor stimulation induces vasorelaxation mediated essentially by endothelium-derived nitric oxide in rat thoracic aorta. Br J Pharmacol, 128 (1): 69-76. [PMID:10498836]
94. Uehling DE, Shearer BG, Donaldson KH, Chao EY, Deaton DN, Adkison KK, Brown KK, Cariello NF, Faison WL, Lancaster ME et al.. (2006) Biarylaniline phenethanolamines as potent and selective beta3 adrenergic receptor agonists. J Med Chem, 49 (9): 2758-71. [PMID:16640337]
95. Umekawa T, Yoshida T, Sakane N, Kogure A, Kondo M, Honjyo H. (1999) Trp64Arg mutation of beta3-adrenoceptor gene deteriorates lipolysis induced by beta3-adrenoceptor agonist in human omental adipocytes. Diabetes, 48 (1): 117-20. [PMID:9892231]
96. van Wieringen JP, Michel-Reher MB, Hatanaka T, Ueshima K, Michel MC. (2013) The new radioligand [(3)H]-L 748,337 differentially labels human and rat β3-adrenoceptors. Eur J Pharmacol, 720 (1-3): 124-30. [PMID:24183974]
97. Varghese P, Harrison RW, Lofthouse RA, Georgakopoulos D, Berkowitz DE, Hare JM. (2000) beta(3)-adrenoceptor deficiency blocks nitric oxide-dependent inhibition of myocardial contractility. J Clin Invest, 106 (5): 697-703. [PMID:10974023]
98. Walston J, Silver K, Bogardus C, Knowler WC, Celi FS, Austin S, Manning B, Strosberg AD, Stern MP, Raben N, et al.. (1995) Time of onset of non-insulin-dependent diabetes mellitus and genetic variation in the beta 3-adrenergic-receptor gene. N Engl J Med, 333: 343-347. [PMID:7609750]
99. Wang HD, Zhang CS, Li MW, Lin Q, Zhang Q, Liu DF, Ma ZY, Dong J. (2021) The Association of Trp64Arg Polymorphism in the Beta-Adrenergic Receptor With Insulin Resistance: Meta-Analysis. Front Endocrinol (Lausanne), 12: 708139. [PMID:34512548]
100. Weber AE, Ok HO, Alvaro RF, Candelore MR, Cascieri MA, Chiu SH, Deng L, Forrest MJ, Hom GJ, Hutchins JE et al.. (1998) 3-Pyridyloxypropanolamine agonists of the beta 3 adrenergic receptor with improved pharmacokinetic properties. Bioorg Med Chem Lett, 8 (16): 2111-6. [PMID:9873496]
101. Yanagisawa T, Sato T, Yamada H, Sukegawa J, Nunoki K. (2000) Selectivity and potency of agonists for the three subtypes of cloned human beta-adrenoceptors expressed in Chinese hamster ovary cells. Tohoku J Exp Med, 192 (3): 181-93. [PMID:11249148]
102. Yoneshiro T, Ogawa T, Okamoto N, Matsushita M, Aita S, Kameya T, Kawai Y, Iwanaga T, Saito M. (2013) Impact of UCP1 and β3AR gene polymorphisms on age-related changes in brown adipose tissue and adiposity in humans. Int J Obes (Lond), 37 (7): 993-8. [PMID:23032405]
103. Zhang Y, Wat N, Stratton IM, Warren-Perry MG, Orho M, Groop L, Turner RC. (1996) UKPDS 19: heterogeneity in NIDDM: separate contributions of IRS-1 and beta 3-adrenergic-receptor mutations to insulin resistance and obesity respectively with no evidence for glycogen synthase gene mutations. UK Prospective Diabetes Study. Diabetologia, 39 (12): 1505-11. [PMID:8960833]
104. Zilberfarb V, Piétri-Rouxel F, Jockers R, Krief S, Delouis C, Issad T, Strosberg AD. (1997) Human immortalized brown adipocytes express functional beta3-adrenoceptor coupled to lipolysis. J Cell Sci, 110 ( Pt 7): 801-7. [PMID:9133667]














