GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
Contents:
Gene and Protein Information  |
||||||
| Species | TM | AA | Chromosomal Location | Gene Symbol | Gene Name | Reference |
| Human | - | 522 | 8p12 | GSR | glutathione-disulfide reductase | |
| Mouse | - | 500 | 8 20.69 cM | Gsr | glutathione reductase | |
| Rat | - | 424 | 16q12.3 | Gsr | glutathione-disulfide reductase | |
Previous and Unofficial Names  |
| glutathione reductase, mitochondrial | glutathione reductase |
Database Links  |
|
| Alphafold | P00390 (Hs), P47791 (Mm), P70619 (Rn) |
| BRENDA | 1.8.1.7 |
| CATH/Gene3D | 3.50.50.60, 3.30.390.30 |
| ChEMBL Target | CHEMBL2755 (Hs), CHEMBL2366476 (Mm), CHEMBL3286088 (Rn) |
| DrugBank Target | P00390 (Hs) |
| Ensembl Gene | ENSG00000104687 (Hs), ENSMUSG00000031584 (Mm), ENSRNOG00000014915 (Rn) |
| Entrez Gene | 2936 (Hs), 14782 (Mm), 116686 (Rn) |
| Human Protein Atlas | ENSG00000104687 (Hs) |
| KEGG Enzyme | 1.8.1.7 |
| KEGG Gene | hsa:2936 (Hs), mmu:14782 (Mm), rno:116686 (Rn) |
| OMIM | 138300 (Hs) |
| Orphanet | ORPHA122298 (Hs) |
| Pharos | P00390 (Hs) |
| RefSeq Nucleotide | NM_000637 (Hs), NM_010344 (Mm), NM_053906 (Rn) |
| RefSeq Protein | NP_000628 (Hs), NP_034474 (Mm), NP_446358 (Rn) |
| SynPHARM | 78934 (in complex with oxiglutatione) |
| UniProtKB | P00390 (Hs), P47791 (Mm), P70619 (Rn) |
| Wikipedia | GSR (Hs) |
Selected 3D Structures  |
|||||||||||
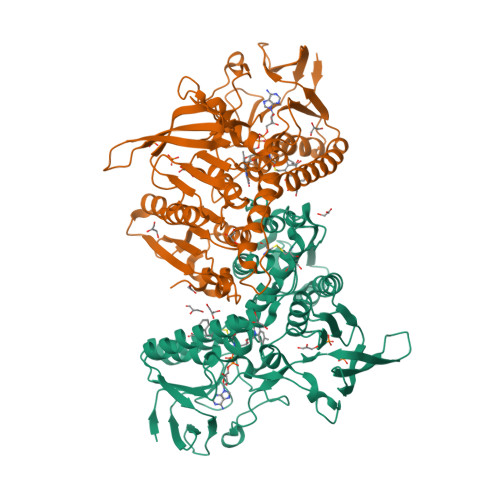
|
|
||||||||||
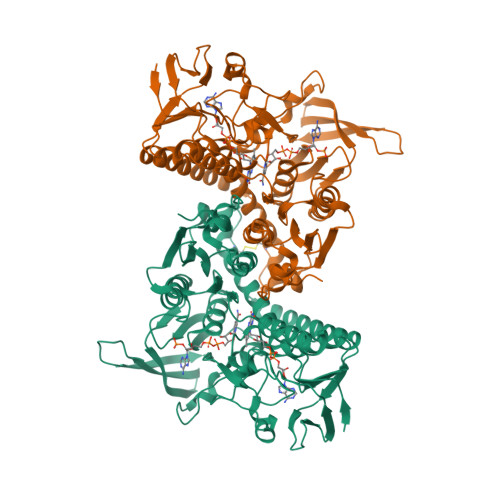
|
|
||||||||||
Enzyme Reaction  |
||||
|
||||
Download all structure-activity data for this target as a CSV file 
| Inhibitors | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| View species-specific inhibitor tables | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Inhibitor Comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Apart from the CHEMBL listing of oxiglutatione as an approved drug acting at glutathione reductase and one PubMed reference describing a its use in a single clinical case study [4], there is very little information available about this drug and its mechanism of action. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Clinically-Relevant Mutations and Pathophysiology 
|
||||||||
|
||||||||
References
1. Bauer H, Fritz-Wolf K, Winzer A, Kühner S, Little S, Yardley V, Vezin H, Palfey B, Schirmer RH, Davioud-Charvet E. (2006) A fluoro analogue of the menadione derivative 6-[2'-(3'-methyl)-1',4'-naphthoquinolyl]hexanoic acid is a suicide substrate of glutathione reductase. Crystal structure of the alkylated human enzyme. J Am Chem Soc, 128 (33): 10784-94. [PMID:16910673]
2. Berkholz DS, Faber HR, Savvides SN, Karplus PA. (2008) Catalytic cycle of human glutathione reductase near 1 A resolution. J Mol Biol, 382 (2): 371-84. [PMID:18638483]
3. Liu X, Sturla SJ. (2009) Profiling patterns of glutathione reductase inhibition by the natural product illudin S and its acylfulvene analogues. Mol Biosyst, 5 (9): 1013-24. [PMID:19668867]
4. Nakatani Y, Nishimura A, Sugiyama K. (2013) Successful treatment of corneal wasp sting-induced panuveitis with vitrectomy. J Ophthalmic Inflamm Infect, 3 (1): 18. [PMID:23514564]
How to cite this page
1.-.-.- Oxidoreductases: glutathione-disulfide reductase. Last modified on 13/08/2015. Accessed on 28/01/2026. IUPHAR/BPS Guide to PHARMACOLOGY, https://www.guidetoimmunopharmacology.org/GRAC/ObjectDisplayForward?objectId=2613.









