GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
Contents:
Gene and Protein Information  |
||||||
| Species | TM | AA | Chromosomal Location | Gene Symbol | Gene Name | Reference |
| Human | - | 604 | 1q31.1 | PTGS2 | prostaglandin-endoperoxide synthase 2 | |
| Mouse | - | 604 | 1 63.84 cM | Ptgs2 | prostaglandin-endoperoxide synthase 2 | |
| Rat | - | 604 | 13q21 | Ptgs2 | prostaglandin-endoperoxide synthase 2 | |
Database Links  |
|
| Alphafold | P35354 (Hs), Q05769 (Mm), P35355 (Rn) |
| BRENDA | 1.14.99.1 |
| CATH/Gene3D | 1.10.640.10 |
| ChEMBL Target | CHEMBL230 (Hs), CHEMBL4321 (Mm), CHEMBL2977 (Rn) |
| DrugBank Target | P35354 (Hs) |
| Ensembl Gene | ENSG00000073756 (Hs), ENSMUSG00000032487 (Mm), ENSRNOG00000002525 (Rn) |
| Entrez Gene | 5743 (Hs), 19225 (Mm), 29527 (Rn) |
| Human Protein Atlas | ENSG00000073756 (Hs) |
| KEGG Enzyme | 1.14.99.1 |
| KEGG Gene | hsa:5743 (Hs), mmu:19225 (Mm), rno:29527 (Rn) |
| OMIM | 600262 (Hs) |
| Pharos | P35354 (Hs) |
| SynPHARM |
79412 (in complex with meclofenamic acid) 79499 (in complex with mefenamic acid) |
| UniProtKB | P35354 (Hs), Q05769 (Mm), P35355 (Rn) |
| Wikipedia | PTGS2 (Hs) |
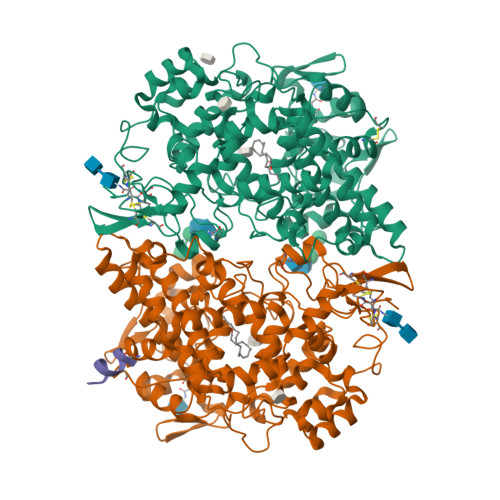
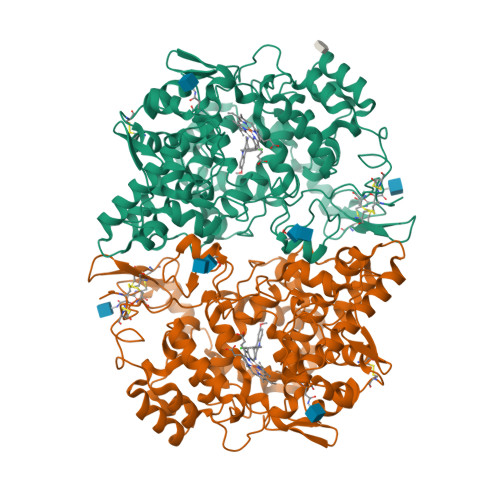
Selected 3D Structures  |
|||||||||||||
|
|
||||||||||||
|
|
||||||||||||
Enzyme Reaction  |
|||||||||||||
|
|||||||||||||
Download all structure-activity data for this target as a CSV file 
| Inhibitors | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| View species-specific inhibitor tables | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Inhibitor Comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Carprofen is a COX-2 selective cyclooxygenase inhibitor, at least when comparing human COX-2 vs. ovine COX-1 in the same assay [13-14]. The data for etodolac in the table above is from Kato et al. (2001) [21]. In the same study etodolac's IC50 for COX-1 was reported to be >100 μM. Piroxicam inhibits both cyclooxygenase isozymes [27], with maximum inhibition of PGE2 synthesis of approximately 60% for COX-2 and 35% for COX-1. Ketorolac is also a non-selective COX inhibitor. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Immunopharmacology Comments |
| The cyclooxygenase enzymes are included in GtoImmuPdb as they are involved in the production of inflammatory mediators, and are long-standing anti-inflammatory drug targets. The role of COX-2 in immuno-oncology is reviewed in [1]. |
References
1. Adams JL, Smothers J, Srinivasan R, Hoos A. (2015) Big opportunities for small molecules in immuno-oncology. Nat Rev Drug Discov, 14 (9): 603-22. [PMID:26228631]
2. Auerbach SS, DrugMatrix® and ToxFX® Coordinator National Toxicology Program. National Toxicology Program: Dept of Health and Human Services. Accessed on 02/05/2014. Modified on 02/05/2014. DrugMatrix, https://ntp.niehs.nih.gov/drugmatrix/index.html
3. Bayly CI, Black WC, Léger S, Ouimet N, Ouellet M, Percival MD. (1999) Structure-based design of COX-2 selectivity into flurbiprofen. Bioorg Med Chem Lett, 9 (3): 307-12. [PMID:10091674]
4. Beswick P, Bingham S, Bountra C, Brown T, Browning K, Campbell I, Chessell I, Clayton N, Collins S, Corfield J et al.. (2004) Identification of 2,3-diaryl-pyrazolo[1,5-b]pyridazines as potent and selective cyclooxygenase-2 inhibitors. Bioorg Med Chem Lett, 14 (21): 5445-8. [PMID:15454242]
5. Black WC, Brideau C, Chan CC, Charleson S, Cromlish W, Gordon R, Grimm EL, Hughes G, Leger S, Li CS et al.. (2003) 3,4-Diaryl-5-hydroxyfuranones: highly selective inhibitors of cyclooxygenase-2 with aqueous solubility. Bioorg Med Chem Lett, 13 (6): 1195-8. [PMID:12643942]
6. Blobaum AL, Marnett LJ. (2007) Molecular determinants for the selective inhibition of cyclooxygenase-2 by lumiracoxib. J Biol Chem, 282 (22): 16379-90. [PMID:17434872]
7. Blobaum AL, Marnett LJ. (2007) Structural and functional basis of cyclooxygenase inhibition. J Med Chem, 50 (7): 1425-41. [PMID:17341061]
8. Bézière N, Goossens L, Pommery J, Vezin H, Touati N, Hénichart JP, Pommery N. (2008) New NSAIDs-NO hybrid molecules with antiproliferative properties on human prostatic cancer cell lines. Bioorg Med Chem Lett, 18 (16): 4655-7. [PMID:18667313]
9. Geisslinger G, Schaible HG. (1996) New insights into the site and mode of antinociceptive action of flurbiprofen enantiomers. J Clin Pharmacol, 36 (6): 513-20. [PMID:8809636]
10. Geusens P. (2009) Naproxcinod, a new cyclooxygenase-inhibiting nitric oxide donator (CINOD). Expert Opin Biol Ther, 9 (5): 649-57. [PMID:19392579]
11. Gierse JK, McDonald JJ, Hauser SD, Rangwala SH, Koboldt CM, Seibert K. (1996) A single amino acid difference between cyclooxygenase-1 (COX-1) and -2 (COX-2) reverses the selectivity of COX-2 specific inhibitors. J Biol Chem, 271 (26): 15810-4. [PMID:8663121]
12. Heinrich DM, Flanagan JU, Jamieson SM, Silva S, Rigoreau LJ, Trivier E, Raynham T, Turnbull AP, Denny WA. (2013) Synthesis and structure-activity relationships for 1-(4-(piperidin-1-ylsulfonyl)phenyl)pyrrolidin-2-ones as novel non-carboxylate inhibitors of the aldo-keto reductase enzyme AKR1C3. Eur J Med Chem, 62: 738-44. [PMID:23454516]
13. Hieke M, Ness J, Steri R, Dittrich M, Greiner C, Werz O, Baumann K, Schubert-Zsilavecz M, Weggen S, Zettl H. (2010) Design, synthesis, and biological evaluation of a novel class of gamma-secretase modulators with PPARgamma activity. J Med Chem, 53 (12): 4691-700. [PMID:20503989]
14. Hieke M, Ness J, Steri R, Greiner C, Werz O, Schubert-Zsilavecz M, Weggen S, Zettl H. (2011) SAR studies of acidic dual γ-secretase/PPARγ modulators. Bioorg Med Chem, 19 (18): 5372-82. [PMID:21873070]
15. Hinz B, Cheremina O, Brune K. (2008) Acetaminophen (paracetamol) is a selective cyclooxygenase-2 inhibitor in man. FASEB J, 22 (2): 383-90. [PMID:17884974]
16. Hoshino J, Park EJ, Kondratyuk TP, Marler L, Pezzuto JM, van Breemen RB, Mo S, Li Y, Cushman M. (2010) Selective synthesis and biological evaluation of sulfate-conjugated resveratrol metabolites. J Med Chem, 53 (13): 5033-43. [PMID:20527891]
17. Imanishi J, Morita Y, Yoshimi E, Kuroda K, Masunaga T, Yamagami K, Kuno M, Hamachi E, Aoki S, Takahashi F et al.. (2011) Pharmacological profile of FK881(ASP6537), a novel potent and selective cyclooxygenase-1 inhibitor. Biochem Pharmacol, 82 (7): 746-54. [PMID:21745460]
18. Inagaki M, Tsuri T, Jyoyama H, Ono T, Yamada K, Kobayashi M, Hori Y, Arimura A, Yasui K, Ohno K et al.. (2000) Novel antiarthritic agents with 1,2-isothiazolidine-1,1-dioxide (gamma-sultam) skeleton: cytokine suppressive dual inhibitors of cyclooxygenase-2 and 5-lipoxygenase. J Med Chem, 43 (10): 2040-8. [PMID:10821716]
19. Kalgutkar AS, Rowlinson SW, Crews BC, Marnett LJ. (2002) Amide derivatives of meclofenamic acid as selective cyclooxygenase-2 inhibitors. Bioorg Med Chem Lett, 12 (4): 521-4. [PMID:11844663]
20. Kassab SE, Khedr MA, Ali HI, Abdalla MM. (2017) Discovery of new indomethacin-based analogs with potentially selective cyclooxygenase-2 inhibition and observed diminishing to PGE2 activities. Eur J Med Chem, 141: 306-321. [PMID:29031075]
21. Kato M, Nishida S, Kitasato H, Sakata N, Kawai S. (2001) Cyclooxygenase-1 and cyclooxygenase-2 selectivity of non-steroidal anti-inflammatory drugs: investigation using human peripheral monocytes. J Pharm Pharmacol, 53 (12): 1679-85. [PMID:11804398]
22. Kawai S, Nishida S, Kato M, Furumaya Y, Okamoto R, Koshino T, Mizushima Y. (1998) Comparison of cyclooxygenase-1 and -2 inhibitory activities of various nonsteroidal anti-inflammatory drugs using human platelets and synovial cells. Eur J Pharmacol, 347 (1): 87-94. [PMID:9650852]
23. Kiefer JR, Pawlitz JL, Moreland KT, Stegeman RA, Hood WF, Gierse JK, Stevens AM, Goodwin DC, Rowlinson SW, Marnett LJ et al.. (2000) Structural insights into the stereochemistry of the cyclooxygenase reaction. Nature, 405 (6782): 97-101. [PMID:10811226]
24. Kolasa T, Brooks CD, Rodriques KE, Summers JB, Dellaria JF, Hulkower KI, Bouska J, Bell RL, Carter GW. (1997) Nonsteroidal anti-inflammatory drugs as scaffolds for the design of 5-lipoxygenase inhibitors. J Med Chem, 40 (5): 819-24. [PMID:9057869]
25. Kramer JS, Woltersdorf S, Duflot T, Hiesinger K, Lillich FF, Knöll F, Wittmann SK, Klingler FM, Brunst S, Chaikuad A et al.. (2019) Discovery of the First in Vivo Active Inhibitors of the Soluble Epoxide Hydrolase Phosphatase Domain. J Med Chem, 62 (18): 8443-8460. [PMID:31436984]
26. Kumar R, Saha N, Purohit P, Garg SK, Seth K, Meena VS, Dubey S, Dave K, Goyal R, Sharma SS et al.. (2019) Cyclic enaminone as new chemotype for selective cyclooxygenase-2 inhibitory, anti-inflammatory, and analgesic activities. Eur J Med Chem, 182: 111601. DOI: 10.1016/j.ejmech.2019.111601 [PMID:31445233]
27. Lazer ES, Miao CK, Cywin CL, Sorcek R, Wong HC, Meng Z, Potocki I, Hoermann M, Snow RJ, Tschantz MA et al.. (1997) Effect of structural modification of enol-carboxamide-type nonsteroidal antiinflammatory drugs on COX-2/COX-1 selectivity. J Med Chem, 40 (6): 980-9. [PMID:9083488]
28. Migliore M, Habrant D, Sasso O, Albani C, Bertozzi SM, Armirotti A, Piomelli D, Scarpelli R. (2016) Potent multitarget FAAH-COX inhibitors: Design and structure-activity relationship studies. Eur J Med Chem, 109: 216-37. [PMID:26774927]
29. Nicholson T, Belli A, Lord JM, Hazeldine J. (2024) The impact of trauma relevant concentrations of prostaglandin E2 on the anti-microbial activity of the innate immune system. Front Immunol, 15: 1401185. [PMID:39502706]
30. Ottanà R, Carotti S, Maccari R, Landini I, Chiricosta G, Caciagli B, Vigorita MG, Mini E. (2005) In vitro antiproliferative activity against human colon cancer cell lines of representative 4-thiazolidinones. Part I. Bioorg Med Chem Lett, 15 (17): 3930-3. [PMID:15993594]
31. Penning TD, Talley JJ, Bertenshaw SR, Carter JS, Collins PW, Docter S, Graneto MJ, Lee LF, Malecha JW, Miyashiro JM et al.. (1997) Synthesis and biological evaluation of the 1,5-diarylpyrazole class of cyclooxygenase-2 inhibitors: identification of 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benze nesulfonamide (SC-58635, celecoxib). J Med Chem, 40 (9): 1347-65. [PMID:9135032]
32. Riendeau D, Percival MD, Brideau C, Charleson S, Dubé D, Ethier D, Falgueyret JP, Friesen RW, Gordon R, Greig G et al.. (2001) Etoricoxib (MK-0663): preclinical profile and comparison with other agents that selectively inhibit cyclooxygenase-2. J Pharmacol Exp Ther, 296 (2): 558-66. [PMID:11160644]
33. Riendeau D, Salem M, Styhler A, Ouellet M, Mancini JA, Li CS. (2004) Evaluation of loxoprofen and its alcohol metabolites for potency and selectivity of inhibition of cyclooxygenase-2. Bioorg Med Chem Lett, 14 (5): 1201-3. [PMID:14980665]
34. Singh P, Kaur S, Kaur J, Singh G, Bhatti R. (2016) Rational Design of Small Peptides for Optimal Inhibition of Cyclooxygenase-2: Development of a Highly Effective Anti-Inflammatory Agent. J Med Chem, 59 (8): 3920-34. [PMID:27019010]
35. Smith CJ, Zhang Y, Koboldt CM, Muhammad J, Zweifel BS, Shaffer A, Talley JJ, Masferrer JL, Seibert K, Isakson PC. (1998) Pharmacological analysis of cyclooxygenase-1 in inflammation. Proc Natl Acad Sci USA, 95 (22): 13313-8. [PMID:9789085]
36. Takahashi T, Miyazawa M. (2012) N-Caffeoyl serotonin as selective COX-2 inhibitor. Bioorg Med Chem Lett, 22 (7): 2494-6. [PMID:22386242]
37. Talley JJ, Brown DL, Carter JS, Graneto MJ, Koboldt CM, Masferrer JL, Perkins WE, Rogers RS, Shaffer AF, Zhang YY et al.. (2000) 4-[5-Methyl-3-phenylisoxazol-4-yl]- benzenesulfonamide, valdecoxib: a potent and selective inhibitor of COX-2. J Med Chem, 43 (5): 775-7. [PMID:10715145]
38. Uddin MJ, Xu S, Crews BC, Ghebreselasie K, Banerjee S, Marnett LJ. (2020) Harmaline Analogs as Substrate-Selective Cyclooxygenase-2 Inhibitors. ACS Med Chem Lett, ARTICLES ASAP. DOI: 10.1021/acsmedchemlett.9b00555
39. Viegas A, Manso J, Corvo MC, Marques MM, Cabrita EJ. (2011) Binding of ibuprofen, ketorolac, and diclofenac to COX-1 and COX-2 studied by saturation transfer difference NMR. J Med Chem, 54 (24): 8555-62. [PMID:22091869]
40. Wang JL, Limburg D, Graneto MJ, Springer J, Hamper JR, Liao S, Pawlitz JL, Kurumbail RG, Maziasz T, Talley JJ et al.. (2010) The novel benzopyran class of selective cyclooxygenase-2 inhibitors. Part 2: the second clinical candidate having a shorter and favorable human half-life. Bioorg Med Chem Lett, 20 (23): 7159-63. [PMID:20709553]
41. Warner TD, Giuliano F, Vojnovic I, Bukasa A, Mitchell JA, Vane JR. (1999) Nonsteroid drug selectivities for cyclo-oxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: a full in vitro analysis. Proc Natl Acad Sci USA, 96 (13): 7563-8. [PMID:10377455]
42. Wilkerson WW, Copeland RA, Covington M, Trzaskos JM. (1995) Antiinflammatory 4,5-diarylpyrroles. 2. Activity as a function of cyclooxygenase-2 inhibition. J Med Chem, 38 (20): 3895-901. [PMID:7562922]
43. Zhang Z, Ghosh A, Connolly PJ, King P, Wilde T, Wang J, Dong Y, Li X, Liao D, Chen H et al.. (2021) Gut-Restricted Selective Cyclooxygenase-2 (COX-2) Inhibitors for Chemoprevention of Colorectal Cancer. J Med Chem, 64 (15): 11570-11596. [PMID:34279934]
44. Zhou H, Liu W, Su Y, Wei Z, Liu J, Kolluri SK, Wu H, Cao Y, Chen J, Wu Y et al.. (2010) NSAID sulindac and its analog bind RXRalpha and inhibit RXRalpha-dependent AKT signaling. Cancer Cell, 17 (6): 560-73. [PMID:20541701]

 Target has curated data in GtoImmuPdb
Target has curated data in GtoImmuPdb