GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Compound class:
Synthetic organic
Comment: LW106 is a novel small-molecule IDO1 inhibitor [1]. In xenograft models LW106 dose-dependently inhibited tumour outgrowth more effectively than the existing IDO1 inhibitor epacadostat, despite epacadostat being a more potent inhibitor in vitro [2]. LW106 promoted tumour infiltration by proliferative Teff cells and reduced recruitment of proliferative Treg cells, endothelial cells and cancer-associated fibroblasts. It also reduced tumour resident cancer stem cell numbers.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
Classification  |
|
| Compound class | Synthetic organic |
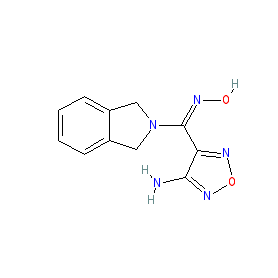
IUPAC Name  |
| 4-[(E)-(2,3-dihydro-1H-isoindol-2-yl)(hydroxyimino)methyl]-1,2,5-oxadiazol-3-amine |
Database Links  |
|
| GtoPdb PubChem SID | 363894234 |
| PubChem CID | 137253428 |
| Search Google for chemical match using the InChIKey | QHVIXMMITPHBSL-ACCUITESSA-N |
| Search Google for chemicals with the same backbone | QHVIXMMITPHBSL |
| UniChem Compound Search for chemical match using the InChIKey | QHVIXMMITPHBSL-ACCUITESSA-N |
| UniChem Connectivity Search for chemical match using the InChIKey | QHVIXMMITPHBSL-ACCUITESSA-N |