GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
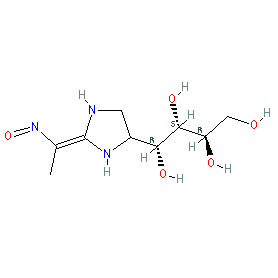
Synonyms: LX3305
Compound class:
Synthetic organic
Comment: LX2931 (LX3305) is a first-in-class, orally available, functional S1P lyase inhibitor, that has been investigated for anti-inflammatory potential [1]. Note that LX2931 does not block S1PL catalytic activity in a biochemical assay even at 100 μM [6].
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Bagdanoff JT, Donoviel MS, Nouraldeen A, Carlsen M, Jessop TC, Tarver J, Aleem S, Dong L, Zhang H, Boteju L et al.. (2010)
Inhibition of sphingosine 1-phosphate lyase for the treatment of rheumatoid arthritis: discovery of (E)-1-(4-((1R,2S,3R)-1,2,3,4-tetrahydroxybutyl)-1H-imidazol-2-yl)ethanone oxime (LX2931) and (1R,2S,3R)-1-(2-(isoxazol-3-yl)-1H-imidazol-4-yl)butane-1,2,3,4-tetraol (LX2932). J Med Chem, 53 (24): 8650-62. [PMID:21090716] |
|
2. Bagdanoff JT, Donoviel MS, Nouraldeen A, Tarver J, Fu Q, Carlsen M, Jessop TC, Zhang H, Hazelwood J, Nguyen H et al.. (2009)
Inhibition of sphingosine-1-phosphate lyase for the treatment of autoimmune disorders. J Med Chem, 52 (13): 3941-53. [PMID:19489538] |
|
3. Billich A, Beerli C, Bergmann R, Bruns C, Loetscher E. (2013)
Cellular assay for the characterization of sphingosine-1-phosphate lyase inhibitors. Anal Biochem, 434 (2): 247-53. [PMID:23246729] |
|
4. Loetscher E, Schneider K, Beerli C, Billich A. (2013)
Assay to measure the secretion of sphingosine-1-phosphate from cells induced by S1P lyase inhibitors. Biochem Biophys Res Commun, 433 (3): 345-8. [PMID:23499842] |
|
5. Schwab SR, Pereira JP, Matloubian M, Xu Y, Huang Y, Cyster JG. (2005)
Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science, 309 (5741): 1735-9. [PMID:16151014] |
|
6. Weiler S, Braendlin N, Beerli C, Bergsdorf C, Schubart A, Srinivas H, Oberhauser B, Billich A. (2014)
Orally active 7-substituted (4-benzylphthalazin-1-yl)-2-methylpiperazin-1-yl]nicotinonitriles as active-site inhibitors of sphingosine 1-phosphate lyase for the treatment of multiple sclerosis. J Med Chem, 57 (12): 5074-84. [PMID:24809814] |
|
7. Yu XQ, Kramer J, Moran L, O'Neill E, Nouraldeen A, Oravecz T, Wilson AG. (2010)
Pharmacokinetic/pharmacodynamic modelling of 2-acetyl-4(5)-tetrahydroxybutyl imidazole-induced peripheral lymphocyte sequestration through increasing lymphoid sphingosine 1-phosphate. Xenobiotica, 40 (5): 350-6. [PMID:20175664] |