GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
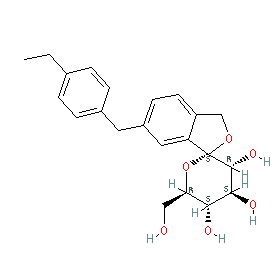
Synonyms: Apleway® | compound 16d [PMID: 22889351] | CSG-452 | CSG452 | Deberza®
tofogliflozin is an approved drug (Japan (2014))
Compound class:
Synthetic organic
Comment: Tofogliflozin is a selective sodium/glucose cotransporter 2 (SGLT2; SLC5A2) inhibitor [2] (a 'gliflozin' family drug [1]) developed as a treatment for type 2 diabetes. We show the anhydrous structure as represented in the INN record for tofogliflozin. In practice the drug is administered as the monohydrate (PubChem CID 46908928).
Tofogliflozin has favourable oral bioavailability and pharmacokinetic profile [2]. Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Approved in Japan for the treatment of type 2 diabetes [3]. This approval permits tofogliflozin to be prescribed either as monotherapy or in combination with other antihyperglcaemic agents. Post-approval monitoring as an add-on treatment to insulin is being provided by Phase 4 clinical trial NCT02201004. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT02201004 | TOFO Insulin Combination Trial | Phase 4 Interventional | Sanofi | ||