GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
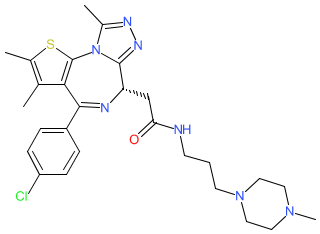
Synonyms: (S)-JQ-35 | (S)-JQ35 | RG6146 | Ro-6870810 | RO-6870810 | RO6870810 | TEN010
Compound class:
Synthetic organic
Comment: TEN-010 (RO6870810) is a BET inhibitor [3]. Structurally it is an analogue of (+)-JQ1, with optimised chemical and biological properties. Development of TEN-010 was progressed by Roche, who acquired the originating biotech firm Tensha Therapeutics. Tensha claimed several bromodomain inhibitors in patent WO2016069578 [1].
BET inhibitors bind to the bromodomains of the bromodomain and extra-terminal motif (BET) proteins BRD2, BRD3, BRD4, and BRDT. This mechanism acts to prevent protein-protein interaction between BET proteins and acetylated histones and transcription factors. The action disrupts chromatin remodeling and inhibits expression of some growth-promoting genes which slows proliferation in susceptible tumours. |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| TEN-010 is being evaluated in Phase 1 clinical trials: NCT01987362 in patients with solid tumours and NCT02308761 in patients with acute myeloid leukemia (AML) and myelodysplastic syndrome. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT02308761 | A Study of RO6870810/TEN-010 in Participants With Acute Myeloid Leukemia and Myelodysplastic Syndrome | Phase 1 Interventional | Hoffmann-La Roche | 2 | |
| NCT01987362 | A Two Part Study of RO6870810. Dose-Escalation Study in Participants With Advanced Solid Tumors and Expansion Study in Participants With Selected Malignancies | Phase 1 Interventional | Hoffmann-La Roche | 3 | |