GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
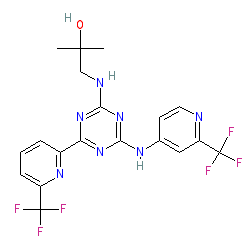
Synonyms: AG-221 | CC-90007 | Idhifa®
enasidenib is an approved drug (FDA (2017))
Compound class:
Synthetic organic
Comment: Enasidenib (AG-221) is an orally available inhibitor of isocitrate dehygrogenase 2 (IDH2), that is approved as an antineoplastic agent. This compound is example 409 in patent US20130190287 A1 [1].
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Having completed Phase 3 clinical evaluation, enasidenib (AG-221) was approved by the US FDA in August 2017 [2], for the treatment for IDH2 mutation positive acute myeloid leukemia (AML- see trial NCT02577406). The presence of IDH2 mutations must be confirmed using the companion diagnostic, RealTime IDH2 Assay. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT02577406 | An Efficacy and Safety Study of AG-221 (CC-90007) Versus Conventional Care Regimens in Older Subjects With Late Stage Acute Myeloid Leukemia Harboring an Isocitrate Dehydrogenase 2 Mutation | Phase 3 Interventional | Celgene | ||