GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Compound class:
Natural product
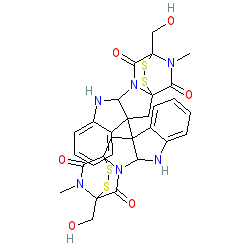
Comment: Chaetocin is a fungal mycotoxin specifically inhibiting the histone methyltransferase (HMT), suppressor of variegation 3-9 homolog 1 (SUV39H1) [2-3]. SUV39H1 is the main enzyme responsible for the trimethylation of lysine 9 in histone H3.
We show the chemical structure here without any stereochemical specification. PubChem records various stereoisomers of this naturally ocurring metabolite. Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Chaib H, Nebbioso A, Prebet T, Castellano R, Garbit S, Restouin A, Vey N, Altucci L, Collette Y. (2012)
Anti-leukemia activity of chaetocin via death receptor-dependent apoptosis and dual modulation of the histone methyl-transferase SUV39H1. Leukemia, 26 (4): 662-74. [PMID:21979880] |
|
2. Cherblanc FL, Chapman KL, Brown R, Fuchter MJ. (2013)
Chaetocin is a nonspecific inhibitor of histone lysine methyltransferases. Nat Chem Biol, 9 (3): 136-7. [PMID:23416387] |
|
3. Greiner D, Bonaldi T, Eskeland R, Roemer E, Imhof A. (2005)
Identification of a specific inhibitor of the histone methyltransferase SU(VAR)3-9. Nat Chem Biol, 1 (3): 143-5. [PMID:16408017] |
|
4. Isham CR, Tibodeau JD, Jin W, Xu R, Timm MM, Bible KC. (2007)
Chaetocin: a promising new antimyeloma agent with in vitro and in vivo activity mediated via imposition of oxidative stress. Blood, 109 (6): 2579-88. [PMID:17090648] |