GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
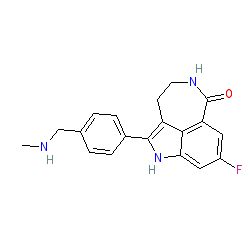
Synonyms: AG-014699 | AG014699 | AG14447 | PF-01367338 | Rubraca®
rucaparib is an approved drug (FDA (2016), EMA (2018))
Compound class:
Synthetic organic
Comment: Rucaparib is an orally active poly(ADP-ribose)polymerase (PARP) inhibitor with anti-cancer activity [7]. It was developed by Clovis Oncology.
PARP inhibitors are known to enhance efficacy of anti-cancer therapies such as DNA alkylating agents, topoisomerase I poisons, and ionizing radiation. Note that in the Thomas et al. paper [7], AG014699 refers to the phosphate salt and AG14447 to the parent molecule. Rucaparib was accepted for FDA priority review for the treatment of advanced ovarian cancer harbouring mutant BRCA (August 2016). Note: olaparib was the first PARP inhibitor to be approved for clinical use. Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Abida W, Campbell D, Patnaik A, Shapiro JD, Sautois B, Vogelzang NJ, Voog EG, Bryce AH, McDermott R, Ricci F et al.. (2020)
Non-BRCA DNA Damage Repair Gene Alterations and Response to the PARP Inhibitor Rucaparib in Metastatic Castration-Resistant Prostate Cancer: Analysis From the Phase II TRITON2 Study. Clin Cancer Res, 26 (11): 2487-2496. [PMID:32086346] |
|
2. Calabrese CR, Almassy R, Barton S, Batey MA, Calvert AH, Canan-Koch S, Durkacz BW, Hostomsky Z, Kumpf RA, Kyle S et al.. (2004)
Anticancer chemosensitization and radiosensitization by the novel poly(ADP-ribose) polymerase-1 inhibitor AG14361. J Natl Cancer Inst, 96 (1): 56-67. [PMID:14709739] |
|
3. Coleman RL, Oza AM, Lorusso D, Aghajanian C, Oaknin A, Dean A, Colombo N, Weberpals JI, Clamp A, Scambia G et al.. (2017)
Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet, 390 (10106): 1949-1961. [PMID:28916367] |
|
4. Delaney CA, Wang LZ, Kyle S, White AW, Calvert AH, Curtin NJ, Durkacz BW, Hostomsky Z, Newell DR. (2000)
Potentiation of temozolomide and topotecan growth inhibition and cytotoxicity by novel poly(adenosine diphosphoribose) polymerase inhibitors in a panel of human tumor cell lines. Clin Cancer Res, 6 (7): 2860-7. [PMID:10914735] |
|
5. EMA.
Rubraca. Accessed on 07/06/2018. Modified on 07/06/2018. European Medicines Agency, http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/004272/human_med_002215.jsp&mid=WC0b01ac058001d124 |
|
6. Swisher EM, Lin KK, Oza AM, Scott CL, Giordano H, Sun J, Konecny GE, Coleman RL, Tinker AV, O'Malley DM et al.. (2017)
Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): an international, multicentre, open-label, phase 2 trial. Lancet Oncol, 18 (1): 75-87. [PMID:27908594] |
|
7. Thomas HD, Calabrese CR, Batey MA, Canan S, Hostomsky Z, Kyle S, Maegley KA, Newell DR, Skalitzky D, Wang LZ et al.. (2007)
Preclinical selection of a novel poly(ADP-ribose) polymerase inhibitor for clinical trial. Mol Cancer Ther, 6 (3): 945-56. [PMID:17363489] |