GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
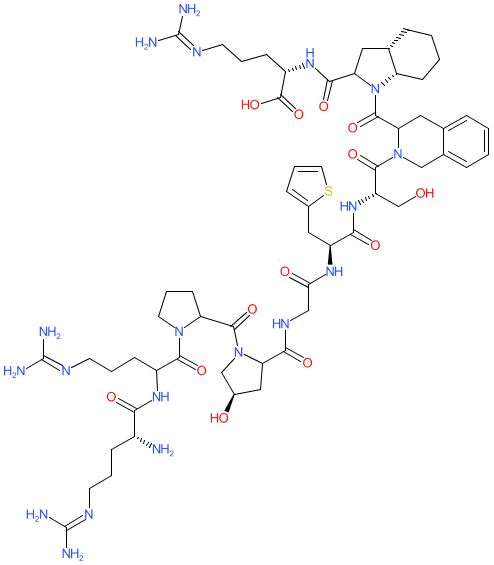
Synonyms: D-Arg-[Hyp3,Thi5,D-Tic7,Oic8]BK | Firazyr® | HOE 140 | HOE140
icatibant is an approved drug (EMA (2008), FDA (2011))
Compound class:
Peptide
Comment: Synthetic analogue of bradykinin. There is some ambiguity in the literature and on online resources as to the exact chemical structure and stereochemistry of icatibant.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Icatibant has been approved to treat hereditary angiodema (HAE). The drug is under investigation for other conditions susceptible to bradykinin amelioration eg ACE inhibitor-induced angiodema, knee ostoearthritis and use during cardiopulmonary bypass surgery. Click here to link to Clinicaltrials.gov's records for this drug. COVID-19: Elevated levels of bradykinin have been detected in COVID-19 patients, and it is proposed that this 'bradykinin storm' might underlie many of the highly damaging symptoms of the infection [2,7,12]. By antagonising bradykinin signalling icatibant provides an exisitng therapeutic option to reduce the multi-system effects of bradykinin [13]. Direct destruction of ACE2 by SARS-CoV-2 binding likely participates in the loss of regulation of bradykinin levels, given ACE2's normal role in degrading vasodilatory des-Arg9-bradykinin [11]. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT04488081 | I-SPY COVID-19 TRIAL: An Adaptive Platform Trial for Critically Ill Patients | Phase 2 Interventional | QuantumLeap Healthcare Collaborative | ||
External links  |
|
For extended ADME data see the following: Electronic Medicines Compendium (eMC) Drugs.com European Medicines Agency (EMA) |