GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
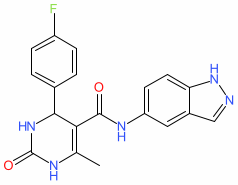
Synonyms: compound 3 [PMID: 17201405] | GSK-180736A
Compound class:
Synthetic organic
Comment: GSK180736A is a Rho-associated coiled-coil kinase 1 (ROCK1) and G protein-coupled receptor kinase 2 (GRK2) inhibitor [1-3].
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Goodman KB, Cui H, Dowdell SE, Gaitanopoulos DE, Ivy RL, Sehon CA, Stavenger RA, Wang GZ, Viet AQ, Xu W et al.. (2007)
Development of dihydropyridone indazole amides as selective Rho-kinase inhibitors. J Med Chem, 50 (1): 6-9. [PMID:17201405] |
|
2. Sehon CA, Wang GZ, Viet AQ, Goodman KB, Dowdell SE, Elkins PA, Semus SF, Evans C, Jolivette LJ, Kirkpatrick RB et al.. (2008)
Potent, selective and orally bioavailable dihydropyrimidine inhibitors of Rho kinase (ROCK1) as potential therapeutic agents for cardiovascular diseases. J Med Chem, 51 (21): 6631-4. [PMID:18842034] |
|
3. Waldschmidt HV, Homan KT, Cruz-Rodríguez O, Cato MC, Waninger-Saroni J, Larimore KM, Cannavo A, Song J, Cheung JY, Kirchhoff PD et al.. (2016)
Structure-Based Design, Synthesis, and Biological Evaluation of Highly Selective and Potent G Protein-Coupled Receptor Kinase 2 Inhibitors. J Med Chem, 59 (8): 3793-807. [PMID:27050625] |
|
4. Xie Z, Yang X, Duan Y, Han J, Liao C. (2021)
Small-Molecule Kinase Inhibitors for the Treatment of Nononcologic Diseases. J Med Chem, 64 (3): 1283-1345. [PMID:33481605] |