GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
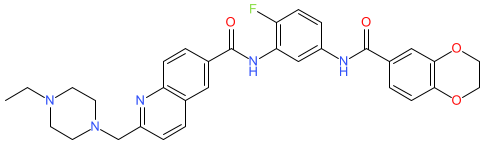
Synonyms: CCT361814 | compound 22 [PMID: 37017629] | NXP-800
Compound class:
Synthetic organic
Comment: NXP800 (CCT361814) was identified as an orally bioavailable heat shock factor 1 (HSF1) pathway inhibitor in a phenotypic screen [3]. It was proposed for anti-proliferative activity in tumours. Subsequent studies identified NXP800 as an activator of the GCN2 (general control nonderepressible 2) kinase that phosphorylates eukaryotic translation initiation factor 2 alpha (EIF2α) [2]. This activity promotes selective ATF4-mediated transcription of genes involved in the integrated stress response (ISR), ultimately leading to apoptosis of tumour cells [4].
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| NXP800 was advanced to clinical evaluation to determine safety and efficacy for the treatment of bile duct cancer (cholangiocarcinoma) [2]. Development for ovarian cancer was discontinued in mid-2025 due to lack of observed efficacy in the phase 1b study. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT06420349 | NXP800 for the Treatment of Patients With Advanced or Metastatic Cholangiocarcinoma | Phase 1 Interventional | Mayo Clinic | 2 | |
| NCT05226507 | A Phase 1 Clinical Study of NXP800 in Subjects with Advanced Cancers and Expansion in Subjects with Ovarian Cancer | Phase 1 Interventional | Nuvectis Pharma, Inc. | ||