GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: compound 19 [WO2023018643A1]
Compound class:
Synthetic organic
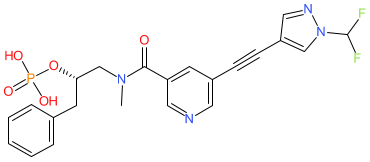
Comment: The chemical structure for fosizensertib was derived from WHO proposed INN list 132 (Feb 2025), in which it is listed as a receptor-interacting serine/threonine protein kinase 1 (RIPK1) inhibitor. This generated a SMILES match via PubChem to a compound (19) claimed in patent WO2023018643A1 (AbbVie) [1]. This appears to be a phosphate prodrug, that is dephosphorylated in vivo, to the active metabolite compound 2. In the absence of formal structure disclosure (March 2025) we hypothesise that this is likely to be the INN for AbbVie's oral ulcerative colitis lead ABBV-668.
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| ABBV-668 is a clinical candidate for the treatment of ulcerative colitis. It offers therapeutic potential for other chronic autoimmune/inflammatory diseases. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT05570006 | Study of ABBV-668 Oral Capsules to Assess Adverse Events and Change in Disease Activity in Adult Participants With Moderate to Severe Ulcerative Colitis | Phase 2 Interventional | AbbVie | ||
| NCT06477926 | A Study to Assess Relative Bioavailability and Food Effect of ABBV-668 Extended-Release Formulations in Adult Participants | Phase 1 Interventional | AbbVie | ||