GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: HSK-16149 | HSK16149
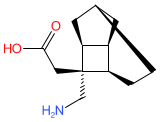
crisugabalin is an approved drug
Compound class:
Synthetic organic
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT06422117 | Efficacy and Safety of HSK16149 Capsule in the Treatment of Moderate and Severe Central Neuropathic Pain in China | Phase 3 Interventional | Haisco Pharmaceutical Group Co., Ltd. | ||
| NCT05890053 | To Evaluate the Long-term Safety and Efficacy of HSK16149 in Chinese Patients With Peripheral Neuralgia | Phase 3 Interventional | Haisco Pharmaceutical Group Co., Ltd. | ||
| NCT06007066 | HSK16149 for Perioperative Analgesia in Orthopedic Surgery | Phase 2 Interventional | Haisco Pharmaceutical Group Co., Ltd. | ||
| NCT04647773 | To Evaluate the Efficacy and Safety of HSK16149 Capsule in Chinese Patients With Diabetic Peripheral Neuropathic Pain | Phase 2/Phase 3 Interventional | Haisco Pharmaceutical Group Co., Ltd. | 2 | |
| NCT05140863 | To Evaluate the Efficacy and Safety of HSK16149 Capsule in Chinese Patients With Postherpetic Neuralgia | Phase 3 Interventional | Haisco Pharmaceutical Group Co., Ltd. | 3 | |