GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Compound class:
Synthetic organic
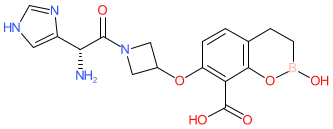
Comment: KSP-1007 is a bicyclic boronic acid β-lactamase inhibitor (BLI), with broad-spectrum activity against both serine and metallo-β-lactamases, discovered through a collaboration between the Kitasato Institute (Tokyo, Japan) and Sumitomo Pharma (Osaka, Japan) [1]. KSP-1007, in combination with meropenem, is under clinical development for the treatment of infections caused by carbapenem-resistant bacterial infections.
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| KSP-1007 is administered intravenously. In September 2022, KSP-1007 in combination with meropenem, was granted Qualified Infectious Disease Product (QIDP) and Fast Track Designation by the US FDA for the treatment of hospital acquired pneumonia (HAP) including ventilator-associated bacterial pneumonia (VAP), complicated urinary tract infections and complicated intra-abdominal infections. A Phase 1 study (NCT05226923) to assess the safety, tolerability, and pharmacokinetics of KSP-1007, alone and co-administered with meropenem, has been completed. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT05226923 | Study of the Safety and Pharmacokinetics of KSP-1007 Alone and Coadministered With Meropenem in Healthy Subjects | Phase 1 Interventional | Sumitovant Biopharma, Inc. | ||