GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: RO-0622
Compound class:
Synthetic organic
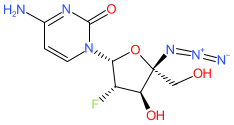
Comment: Azvudine is a nucleoside analogue that was first identified as an inhibitor of the HIV-1 RNA-dependent RNA polymerase (RdRp) [4]. It has more recently been reported to inhibit RdRp from the SARS-CoV-2 betacoronavirus [5]. The active triphosphate metabolite concentrates in the thymus and peripheral blood mononuclear cells, and oral treatment induces curative mechanisms in preclinical models.
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Azvudine was advanced to clinical evaluation for efficacy in the treatment of SARS-CoV-2 infection (COVID-19). |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT05689034 | Multicenter Randomized Double-blind Placebo-controlled Study to Investigate Azvudine in Symptomatic Adults With COVID-19 at Increased Risk of Progressing to Severe Illness | Phase 2/Phase 3 Interventional | Peking Union Medical College Hospital | ||
| NCT05033145 | Study on Safety and Clinical Efficacy of AZVUDINE in Initial Stage COVID-19 Patients (SARS-CoV-2 Infected) | Phase 3 Interventional | HRH Pharmaceuticals Limited | In patients with mild COVID-19 symptoms, azvudine treatment accelerated the elimination of the virus and hastened recovery. | 2 |
| NCT04668235 | Study on Safety and Clinical Efficacy of AZVUDINE in COVID-19 Patients (SARS-CoV-2 Infected) | Phase 3 Interventional | HRH Pharmaceuticals Limited | 3 | |