GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
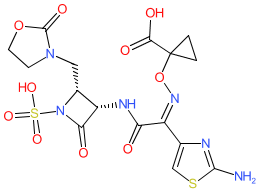
Synonyms: BOS-228 | LYS228
Compound class:
Synthetic organic
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Ancremonam has progressed to Phase 2 clinical evaluation. Studies in patients with complicated intra-abdominal infections (NCT03354754) and complicated urinary tract infections (NCT03377426) were halted due to a change in sponsor. The new sponsor, Boston Pharmaceuticals has indicated that clinical development will continue. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT03377426 | LYS228 PK, Clinical Response, Safety and Tolerability in Patients With Complicated Urinary Tract Infection (cUTI) | Phase 2 Interventional | Novartis | ||
| NCT03354754 | LYS228 PK, Clinical Response, Safety and Tolerability in Patients With Complicated Intra-abdominal Infection (cIAI) | Phase 2 Interventional | Novartis | ||