GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: P005672 | Seysara®
sarecycline is an approved drug (FDA (2018))
Compound class:
Synthetic organic
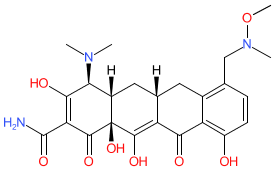
Comment: Sarecycline is a tetracycline antibacterial with narrow-spectrum activity that was developed as an oral anti-acne therapeutic. Note that the structure shown here matches the CAS-assigned structure and is the same as the ChEMBL entry linked to below. The PubChem standardized chemical structure (CID 54681908) for sarecycline is represented with slightly different stereochemistry.
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Sarecycline is indicated for the treatment of moderate to severe cases of inflammatory lesions of non-nodular acne vulgaris. Sarecycline hydrochloride, as an oral formulation under the trade name Seysara®, was granted first approval by the US FDA in October 2018 [1]. |
Mechanism Of Action and Pharmacodynamic Effects  |
| Sarecycline inhibits protein synthesis in the bacterial ribosome and has been shown to bind to two active sites on the 70S ribosome of Cutibacterium acnes [2]. |
External links  |
|
For extended ADME data see the following: Drugs.com |