GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Compound class:
Synthetic organic
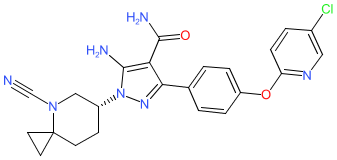
Comment: The structure for civorebrutinib was obtained from proposed INN list 129 (August 2023), where it is described as Bruton's tyrosine kinase inhibitor and antineoplastic agent. It is claimed in patent WO2019091440A1 (SINOMAB Bioscience) [1]. Review of SINOMAB's online pipeline page suggests that civorebrutinib is likely their lead BTK inhibitor SN1011.
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| SN1011 is proposed as a treatment for autoimmune conditions, and has advanced to phase 1 evaluation. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT04041544 | A Study to Evaluate Safety, Tolerability, Pharmacokinetics and Pharmacodynamics in Health Subject | Phase 1 Interventional | SinoMab BioScience Ltd | ||