GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
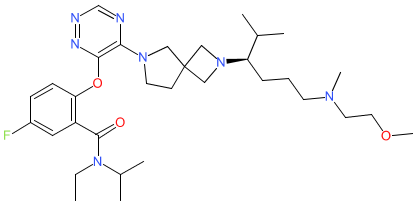
Synonyms: Compound A [WO2022237720] | JNJ-75276617 | JNJ75276617
Compound class:
Synthetic organic
Comment: The structure for bleximenib was obtained from proposed INN list 129 (August 2023). This maps to a PubChem CID that describes it as a menin-mixed-lineage leukemia 1 (menin-MLL) inhibitor. The compound is claimed in patent WO2022237720A1 (Janssen, Johnson & Johnson), for potential to treat hematological malignancies (in combination with a BCL-2 inhibitor) [1]. This class of agents disrupts the interaction between MLL oncoproteins (re-arranged MLL fusion proteins that are found in aggressive human acute leukemias) and the tumour suppressor protein menin (MEN1, O00255). Menin is an essential oncogenic co-factor for the leukemogenicity of MLL fusion proteins. The INN was subsequently mapped to the company code JNJ-75276617 [2].
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Advanced to clinical evaluations in acute leukemias as JNJ-75276617. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT04811560 | A Phase 1/2 Study of Bleximenib in Participants With Acute Leukemia | Phase 1/Phase 2 Interventional | Janssen Research & Development, LLC | ||
| NCT05453903 | A Study of Bleximenib in Combination With Acute Myeloid Leukemia (AML) Directed Therapies | Phase 1 Interventional | Janssen Research & Development, LLC | ||