GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
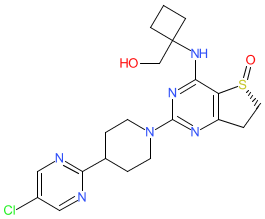
Synonyms: BI 1015550 | BI-1015550 | BI1015550
nerandomilast is an approved drug
Compound class:
Synthetic organic
Comment: BI-1015550 is a PDE4B inhibitor [2]. It is an orally bioavailable compound, and clinical lead for the treatment of idiopathic pulmonary fibrosis. This compound was developed as a possible successor anti-fibrotic mechanism to nintedanib (Ofev) for the treatment of interstitial lung diseases [1,3-4,6]. BI-1015550's chemical structure was matched to the INN nerandomilast from proposed INN list 129 (August 2023).
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT05321082 | A Study to Find Out Whether BI 1015550 Improves Lung Function in People With Progressive Fibrosing Interstitial Lung Diseases (PF-ILDs) | Phase 3 Interventional | Boehringer Ingelheim | The FIBRONEER-ILD trial: Nerandomilast met its primary endpoint in this study (absolute change from baseline in forced vital capacity at Week 52) in patients with progressive pulmonary fibrosis. The results indicate that nerandomilast treatment slows disease progression rather than stopping it, with a modest effect on lung function. | 5 |
| NCT05321069 | A Study to Find Out Whether BI 1015550 Improves Lung Function in People With Idiopathic Pulmonary Fibrosis (IPF) | Phase 3 Interventional | Boehringer Ingelheim | Results from this study (FIBRONEER-IPF trial) show that nerandomilast significantly reduces decline in forced vital capacity (FVC) compared to placebo in patients with IPF at week 52, including in patients on background antifibrotic therapy. | 7 |