GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: Compound 5 [WO2020223685A1]
Compound class:
Synthetic organic
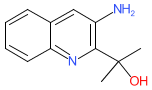
Comment: We obtained the chemical structure for acloproxalap from the WHO's proposed INN list 127 (21 July 2022). In this document it was described as an anti-inflammatory. Acloproxalap is an analogue of reproxalap. Like its predecessor, acloproxalap was designed as a molecular trap for pro-inflammatory Reactive Aldehyde Species (RASP) molecules that are produced by metabolic and inflammatory processes, to treat diseases in which aldehyde toxicity is implicated in the pathogenesis [1]. We predict that acloproxalap is the INN for the clinical lead known as ADX-629.
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| ADX-629 has been advanced to clinical evaluation in several inflammatory conditions, and a small phase 2 study has been completed in COVID-19 patients. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT05443685 | ADX-629 Therapy for Sjogren-Larsson Syndrome | Phase 1/Phase 2 Interventional | University of Nebraska | ||
| NCT05392192 | A Clinical Trial to Evaluate the Safety and Efficacy in Subjects With Chronic Cough | Phase 2 Interventional | Aldeyra Therapeutics, Inc. | ||
| NCT04908514 | A Clinical Trial to Evaluate the Safety, Tolerability, and Efficacy of Subjects With Plaque Psoriasis | Phase 2 Interventional | Aldeyra Therapeutics, Inc. | ||
| NCT04728711 | A Double Masked, Placebo Controlled, Single Center, Randomized Clinical Trial to Assess the Safety and Efficacy of Subjects With Mild Asthma Induced by the Bronchial Allergen Challenge (BAC) | Phase 2 Interventional | Aldeyra Therapeutics, Inc. | ||
| NCT04847544 | A Randomized, Double-Blind, Placebo-Controlled, Clinical Trial to Evaluate the Safety, Tolerability, Efficacy, and Pharmacodynamics for the Treatment of COVID-19. | Phase 2 Interventional | Aldeyra Therapeutics, Inc. | ||