GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
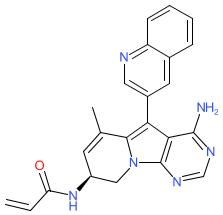
Synonyms: CLN-081 | CLN081 | TAS-6417 | TAS6417
Compound class:
Synthetic organic
Comment: The chemical structure for zipalertinib was revealed in WHO proposed INN list 126 (Jan 2022), in which it was described as a tyrosine kinase inhibitor and antineoplastic agent. Its SMILES matches it to the EGFR inhibitor TAS-6417 (now re-coded as CLN-081) [1] via PubChem. TAS-6417 was designed to selectively target common EGFR mutants in advanced lung cancer [3]. It retains activity against the EGFR with exon 20 insertion mutations, which is a well defined driver of non-small cell lung cancer. It is an orally available compound.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Zipalertinib (CLN-081, and formerly TAS6417) was advanced to clinical trial to evaluate safety and efficacy as a treatment for NSCLC with exon 20 insertion mutation. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT04036682 | A Phase 1/2 Trial of CLN-081 in Patients with Non-Small Cell Lung Cancer | Phase 1/Phase 2 Interventional | Cullinan Therapeutics Inc. | 2 | |
| NCT05967689 | A Study of Zipalertinib in Patients With Advanced Non-Small Cell Lung Cancer With Epidermal Growth Factor Receptor (EGFR) Exon 20 Insertions or Other Uncommon Mutation. | Phase 2 Interventional | Taiho Oncology, Inc. | ||
| NCT05973773 | REZILIENT3 (REsearching ZIpaLertinib In Egfr Non-small Cell Lung Cancer Tumors) | Phase 3 Interventional | Taiho Oncology, Inc. | ||