GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
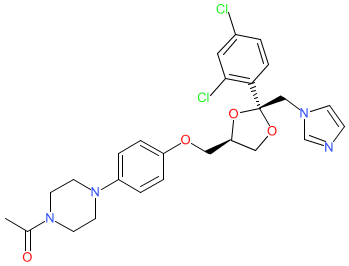
Synonyms: (2S,4R)-ketoconazole | COR-003 | COR003 | Recorlev®
levoketoconazole is an approved drug (FDA (2021))
Compound class:
Synthetic organic
Comment: Levoketoconazole is the pure (2S,4R) enantiomer of the approved drug ketoconazole. Like ketoconazole, levoketoconazole is orally available. It is a more potent inhibitor of glucocorticoid synthesis than the enantiomerically mixed ketoconazole formulation. Levoketoconazole reduces cortisol biosynthesis by inhibiting three key cytochrome P450 enzymes of the glucocorticoid synthesis pathway; 11β-hydroxylase (CYP11B1), 17α-hydroxylase/17,20-lyase (CYP17A1) and steroid 21-hydroxylase (CYP21A2).
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Njar VC, Kato K, Nnane IP, Grigoryev DN, Long BJ, Brodie AM. (1998)
Novel 17-azolyl steroids, potent inhibitors of human cytochrome 17 alpha-hydroxylase-C17,20-lyase (P450(17) alpha): potential agents for the treatment of prostate cancer. J Med Chem, 41 (6): 902-12. [PMID:9526564] |
|
2. Rotstein DM, Kertesz DJ, Walker KA, Swinney DC. (1992)
Stereoisomers of ketoconazole: preparation and biological activity. J Med Chem, 35 (15): 2818-25. [PMID:1495014] |