GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
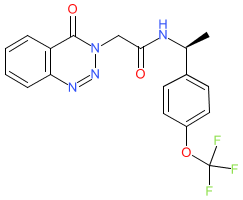
Synonyms: compound 56 [PMID: 34260228] | NBI-1065846 | NBI1065846 | TAK-041 | TAK041
Compound class:
Synthetic organic
Comment: TAK-041 (NBI-1065846) is a clinical stage, orally active GPR139 agonist [1]. Its chemical structure has been matched to the INN zelatriazin.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| TAK-041 is being evaluated as a novel drug to ameliorate the negative symptoms/cognitive impairment of schizophrenia or anhedonia in major depressive disorder (MDD). |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT02748694 | Phase 1 TAK-041 First-in-Human Safety, Tolerability, and Pharmacokinetics Study | Phase 1 Interventional | Neurocrine Biosciences | ||
| NCT03319953 | A Randomized, Double-Blind, Placebo Controlled, Two-Period Cross-Over, Proof of Activity Study to Evaluate the Effects of TAK-041 on Motivational Anhedonia as Add-On to Antipsychotics in Participants With Stable Schizophrenia | Phase 2 Interventional | Neurocrine Biosciences | ||
| NCT02959892 | A Single-Dose Positron Emission Tomography (PET) Study to Determine the Effect of TAK-041 on Amphetamine-Induced Dopamine Release in the Central Nervous System (CNS) | Phase 1 Interventional | Neurocrine Biosciences | ||
| NCT05165394 | Study to Evaluate the Efficacy and Safety of Once-Weekly Oral NBI-1065846 in the Treatment of Anhedonia in MDD | Phase 2 Interventional | Neurocrine Biosciences | ||