GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: Emovit® | ICI-58,834 | ICI-58834 | Qelbree®
viloxazine is an approved drug (FDA (2021))
Compound class:
Synthetic organic
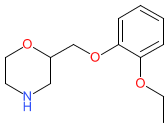
Comment: Viloxazine was originally described as a selective norepinephrine reuptake inhibitor (NRI), principally based on its ability to potentiate noradrenergic effects. It also modulates 5-HT (serotonin) activity, via antagonistic activity at 5-HT2B and agonistic activity at 5-HT2C receptors, and it increases extracellular 5-HT levels in the prefrontal cortex (PFC) [4]. Viloxazine's chemical structure is different from conventional tri- or tetra-cyclic antidepressants. The INN stipulates a racemic mixture of enantiomers. We show the structure without specified stereochemistry to represent the mixture. The hydrocholride salt (SPN-812) was progressed by Supernus Pharmaceuticals for attention deficit hyperactivity disorder (ADHD) in children and adults.
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Viloxazine was used in some European countries as an antidepressant, but it was withdrawn from the market in the early 2000s. The FDA approved extended release viloxazine hydrochloride (Qelbree) as a treatment for ADHD in April 2021 [1]. This is indicated for 6-17 year old children. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT03247530 | Evaluation of SPN-812 ER Low Dose in Children With ADHD | Phase 3 Interventional | Supernus Pharmaceuticals, Inc. | 2-3 | |
| NCT04016779 | Evaluation of SPN-812 in Adults With Attention-Deficit/Hyperactivity Disorder | Phase 3 Interventional | Supernus Pharmaceuticals, Inc. | ||