GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: Azstarys® (serdexmethylphenidate + dexmethylphenidate) | KP-484 | KP484
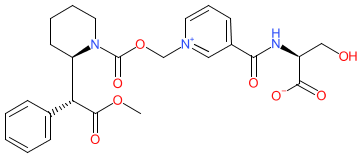
serdexmethylphenidate is an approved drug (FDA (2021))
Compound class:
Synthetic organic
Comment: Serdexmethylphenidate (KP484) is a prodrug that is a slow-release derivative of dexmethylphenidate (d-MPH).
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Serdexmethylphenidate in combination with dexmethylphenidate was approved by the FDA in March 2021 as a treatment for ADHD in children ≥6 years old. The orally administered fixed dose combination was known as KP415 during the development process. Serdexmethylphenidate has a delayed onset and prolonged duration of effects, so acts as a long-acting psychostimulant, whilst dexmethylphenidate provides a more rapid onset of action. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT03460652 | KP415 Open-Label Safety Study in Children (6-12 Years of Age) With ADHD | Phase 3 Interventional | KemPharm, Inc. | ||