GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
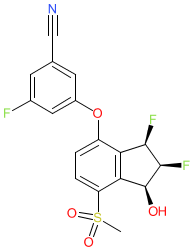
Synonyms: compound 2 [PMID: 31282155] | MK-6482 | MK6482 | PT2977 | Welireg®
belzutifan is an approved drug (FDA (2021))
Compound class:
Synthetic organic
Comment: Belzutifan (previously PT2977 and MK-6482) binds to and inhibits HIF-2α function, and has been developed as a novel cancer therapeutic [4]. HIF-2α is a transcription factor component that is established as an oncogneic driver of ccRCC, via loss of its interaction with the tumour suppressor von Hippel-Lindau (VHL), which results in disrupted proteasomal degradation and thereby accumulation of HIF-2α. Elevated HIF-2α promotes increased expression of hypoxia-responsive genes which regulate processes including angiogenesis, proliferation, migration, and immune evasion.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Belzutifan progressed through advanced clinical evaluation in patients with ccRCC and earlier phase trials in glioblastoma. In August 2021, the FDA approved belzutifan for adult patients with von Hippel-Lindau (VHL) disease, who have VHL-associated cancers (renal cell carcinoma [2,5], central nervous system hemangioblastomas, or pancreatic neuroendocrine tumours) that require therapy. In May 2025 FDA approval was expanded to include treatment of locally advanced, unresectable, or metastatic pheochromocytoma or paraganglioma. Belzutifan was the first oral therapy for these malignancies to reach the clinic. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT03634540 | A Trial of PT2977 in Combination With Cabozantinib in Patients With Clear Cell Renal Cell Carcinoma (ccRCC) | Phase 2 Interventional | Peloton Therapeutics, Inc. | ||
| NCT04195750 | A Study of MK-6482 Versus Everolimus in Participants With Advanced Renal Cell Carcinoma (MK-6482-005) | Phase 3 Interventional | Merck Sharp & Dohme Corp. | ||
| NCT02974738 | A Trial of PT2977 Tablets In Patients With Advanced Solid Tumors | Phase 1 Interventional | Peloton Therapeutics, Inc. | 1 | |
| NCT03401788 | A Phase 2 Study of PT2977 for the Treatment of Von Hippel Lindau Disease-Associated Renal Cell Carcinoma | Phase 2 Interventional | Peloton Therapeutics, Inc. | The LITESPARK-004 study; data were analysed for the CNS haemangioblastomas subgroup, with an additional 16 months of follow-up [3]. | 3 |