GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
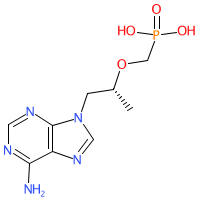
Abbreviated name: TDF
Synonyms: (R)-PMPA | PMPA | tenofovir (anhydrous)
tenofovir is an approved drug
Compound class:
Synthetic organic
Comment: Tenofovir is a nucleotide reverse transcriptase inhibitor (NtRTI) antiretroviral compound. The R-enantiomer, as shown here, is more effective at inhibiting retroviruses than the S-enantiomer [1]. Tenofovir has poor oral bioavailability and is delivered as either the prodrug tenofovir disoproxil fumarate (Viread®) or tenofovir alafenamide fumerate (Vemlidy®).
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Tenofovir is approved for the treatment of HIV (in combination with other antiretroviral agents) and chronic hepatitis B infections. Compared to other nucleotide reverse transcriptase inhibitors, tenofovir demonstrates a favourable safety profile. Renal toxicity has been found to be a modest, but significant risk [2]. SARS-CoV-2 and COVID-19: A number of Phase 2/3 clinical trials are planned to evaluate the use of emtricitabine/tenofovir to prevent SARS-COV-2 transmission to healthcare professionals. |
Mechanism Of Action and Pharmacodynamic Effects  |
| Tenofovir is an acyclic nucleotide diester analogue of adenosine 5'-monophosphate, reverse transciptase inhibitor, preventing viral replication. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT04334928 | Randomized Clinical Trial for the Prevention of SARS-CoV-2 Infection (COVID-19) in Healthcare Personnel | Phase 3 Interventional | Plan Nacional sobre el Sida (PNS) | ||
| NCT04519125 | Daily Regimen of Tenofovir/Emtricitabine as Prevention for COVID-19 in Health Care Personnel in Colombia | Phase 2/Phase 3 Interventional | Hospital Universitario San Ignacio | ||
| NCT04405271 | TAF/FTC for Pre-exposure Prophylaxis of COVID-19 in Healthcare Workers (CoviPrep Study) | Phase 3 Interventional | Hospital Italiano de Buenos Aires | ||
Pharmacokinetics  |
| Biotransformation/Metabolism |
| Bi-phosphorylation to the active compound tenofovir biphosphate. |
| Elimination |
| Tenofovir is eliminated in the urine by glomerular filtration and active tubular secretion. |
| Organ function impairment |
| In the kidney, tenofovir can cause proximal tubule dysfunction leading to Fanconi syndrome, acute tubular necrosis [7] and acute kidney injury (AKI) [5,7]. Severe AKI can cause chronic kidney disease (CKD) and even lead to end stage renal disease (ESRD). Tenofovir can enter mitochondria of tubule cells where it inhibits mitochondrial DNA polymerase γ [3], giving structural mitochondrial abnormalities. This causes apoptosis via mitochondrial protein release. Injured proximal tubule cells are unable to reabsorb small molecules, secrete H+ or synthesize calcitriol [3]. Proximal tubule cell loss is caused from persistent injury, this decreases glomerular filtration rate and injures the kidney. |
External links  |
|
For extended ADME data see the following: Drugs.com |