GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
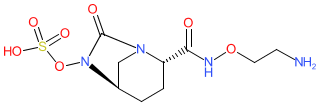
Synonyms: OP-0595 | OP0595 | RG-6080 | RG6080 | RO-7079901 | RO7079901
Compound class:
Synthetic organic
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| The nacubactam + meropenem combination has completed Phase 1 evaluation (NCT03174795). |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT03182504 | A Study to Investigate the Intrapulmonary Lung Penetration of Nacubactam in Healthy Participants | Phase 1 Interventional | Hoffmann-La Roche | ||
| NCT05905055 | P3 Study to Assess Efficacy and Safety of Cefepime/Nacubactam and Aztreonam/Nacubactam Versus Best Available Therapy for Adults With Infection Due to Carbapenem Resistant Enterobacterales | Phase 3 Interventional | Meiji Seika Pharma Co., Ltd. | ||
| NCT05887908 | Efficacy and Safety of Cefepime/Nacubactam or Aztreonam/Nacubactam Compared to Imipenem/Cilastatin in Subjects With Complicated Urinary Tract Infections or Acute Uncomplicated Pyelonephritis | Phase 3 Interventional | Meiji Seika Pharma Co., Ltd. | ||
| NCT03174795 | A Study to Investigate the Pharmacokinetics of RO7079901 and Meropenem in Participants With a Complicated Urinary Tract Infection | Phase 1 Interventional | Hoffmann-La Roche | ||