GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
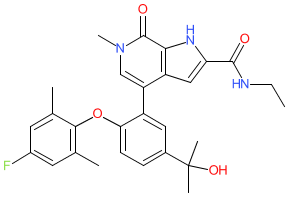
Synonyms: ABBV 744 | ABBV744
Compound class:
Synthetic organic
Comment: ABBV-744 is an investigational, orally bioavailable BET family protein (BRD) inhibitor, that was developed for antiproliferative potential in cancer [1]. It was designed to selectively target the BD2 domain of BET family proteins, in an effort to limit potential on-target adverse activity associated with BD1 interactions. In vivo, ABBV-744 produced fewer platelet and gastrointestinal toxicities than a dual BD1/2-targeting compound in preclinical models. Its antiproliferative effects are primarily evident in acute myeloid leukaemia cell lines and in prostate cancer cells that express the full-length androgen receptor (AR).
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| AbbVie have advanced ABBV-744 to Phase 1 evaluation in advanced prostate cancer and relapsed/refractory acute myeloid leukemia. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT03360006 | A Study Evaluating the Safety and Pharmacokinetics of ABBV-744 in Subjects With Advanced Prostate Cancer (CRPC) and Relapsed/Refractory Acute Myeloid Leukemia (AML) Cancer | Phase 1 Interventional | AbbVie | ||
Pharmacokinetics  |
| Biotransformation/Metabolism |
| ABBV-744 is primarily metabolized by CYP3A4. |