GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
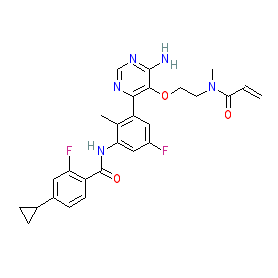
Synonyms: compound 25 [PMID: 32083858] | example 6 [WO2015079417A1] | LOU-064 | LOU064 | LOU064-NXA | NVP-LOU064-NXA | Rhapsido®
remibrutinib is an approved drug
Compound class:
Synthetic organic
Comment: Remibrutinib (LOU064) is one of the examples claimed in Novartis' patent WO2015079417A1 [2], for novel amino pyrimidine derivatives that act as Bruton's tyrosine kinase (BTK) inhibitors and which claims their potential to treat autoimmune disorders, inflammatory/allergic diseases, transplant rejection and cancers of hematopoietic origin or solid tumours. The formal chemical name (LOU064; remibrutinib) to structure disclosure was published in February 2020 [1]. LOU064 is a covalent BTK inhibitor that binds the inactive conformation of the enzyme. This improves its selectivity compared to currently marketed covalent BTK inhibitors, and renders it more suitable for use in non-oncology indications.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT04109313 | Open-label, Multicenter, Extension Study to Evaluate Long-term Safety and Tolerability of LOU064 in Subjects With CSU | Phase 2 Interventional | Novartis | ||
| NCT03944707 | Study of Efficacy and Safety of LOU064 in Inadequately Controlled Asthma Patients | Phase 2 Interventional | Novartis | ||
| NCT04035668 | A Phase 2 Study to Evaluate the Safety and Efficacy of LOU064 in Patients With Moderate to Severe Sjögren's Syndrome | Phase 2 Interventional | Novartis | ||
| NCT03918980 | Single Dose and Multiple Dose Study to Assess Safety and Tolerability of LOU064 | Phase 1 Interventional | Novartis | 4 | |
| NCT03926611 | Dose-finding Study to Evaluate Efficacy and Safety of LOU064 in Patients With CSU Inadequately Controlled by H1-antihistamines | Phase 2 Interventional | Novartis | 5 | |
Pharmacokinetics  |
| Elimination |
| Remibrutinib appears to be eliminated predominantly via the CYP3A4 pathway [3]. |