GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
monoacylglycerol lipase
Target not currently curated in GtoImmuPdb
Target id: 1399
Nomenclature: monoacylglycerol lipase
Abbreviated Name: MAGL
Family: S33: Prolyl aminopeptidase, 2-Acylglycerol ester turnover, Hydrolases & Lipases
Contents:
Gene and Protein Information  |
||||||
| Species | TM | AA | Chromosomal Location | Gene Symbol | Gene Name | Reference |
| Human | - | 303 | 3q21.3 | MGLL | monoglyceride lipase | |
| Mouse | - | 303 | 6 D1 | Mgll | monoglyceride lipase | |
| Rat | - | 303 | 4q34 | Mgll | monoglyceride lipase | |
Previous and Unofficial Names  |
| HU-K5 | MGL | MAGL |
Database Links  |
|
| Specialist databases | |
| MEROPS | S33.980 (Hs) |
| Other databases | |
| Alphafold | Q99685 (Hs), O35678 (Mm), Q8R431 (Rn) |
| BRENDA | 3.1.1.23 |
| CATH/Gene3D | 3.40.50.1820 |
| ChEMBL Target | CHEMBL4191 (Hs), CHEMBL5774 (Mm), CHEMBL3321 (Rn) |
| Ensembl Gene | ENSG00000074416 (Hs), ENSMUSG00000033174 (Mm), ENSRNOG00000014508 (Rn) |
| Entrez Gene | 11343 (Hs), 23945 (Mm), 29254 (Rn) |
| Human Protein Atlas | ENSG00000074416 (Hs) |
| KEGG Enzyme | 3.1.1.23 |
| KEGG Gene | hsa:11343 (Hs), mmu:23945 (Mm), rno:29254 (Rn) |
| OMIM | 609699 (Hs) |
| Pharos | Q99685 (Hs) |
| RefSeq Nucleotide | NM_007283 (Hs), NM_001166251 (Mm), NM_138502 (Rn) |
| RefSeq Protein | NP_009214 (Hs), NP_001159723 (Mm), NP_612511 (Rn) |
| UniProtKB | Q99685 (Hs), O35678 (Mm), Q8R431 (Rn) |
| Wikipedia | MGLL (Hs) |
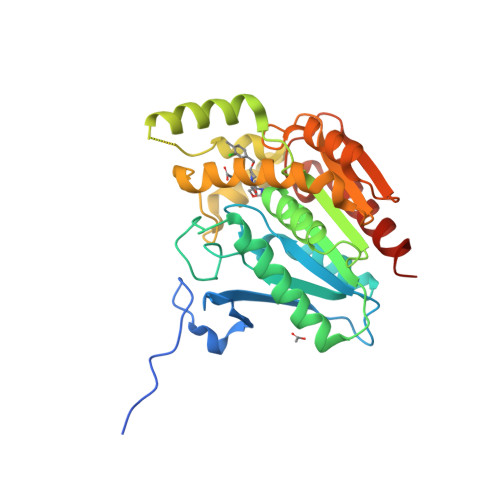
Selected 3D Structures  |
|||||||||||||
|
|
||||||||||||
Enzyme Reaction  |
||||
|
||||
| Endogenous substrates (Human) |
| 2-oleoyl glycerol = 2-arachidonoylglycerol >> anandamide [5] |
Download all structure-activity data for this target as a CSV file 
| Inhibitors | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| View species-specific inhibitor tables | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
1. Aaltonen N, Savinainen JR, Ribas CR, Rönkkö J, Kuusisto A, Korhonen J, Navia-Paldanius D, Häyrinen J, Takabe P, Käsnänen H et al.. (2013) Piperazine and piperidine triazole ureas as ultrapotent and highly selective inhibitors of monoacylglycerol lipase. Chem Biol, 20 (3): 379-90. [PMID:23521796]
2. Chang JW, Niphakis MJ, Lum KM, Cognetta 3rd AB, Wang C, Matthews ML, Niessen S, Buczynski MW, Parsons LH, Cravatt BF. (2012) Highly selective inhibitors of monoacylglycerol lipase bearing a reactive group that is bioisosteric with endocannabinoid substrates. Chem Biol, 19 (5): 579-88. [PMID:22542104]
3. Cisar JS, Weber OD, Clapper JR, Blankman JL, Henry CL, Simon GM, Alexander JP, Jones TK, Ezekowitz RAB, O'Neill GP et al.. (2018) Identification of ABX-1431, a Selective Inhibitor of Monoacylglycerol Lipase and Clinical Candidate for Treatment of Neurological Disorders. J Med Chem, 61 (20): 9062-9084. [PMID:30067909]
4. Clapper JR, Henry CL, Niphakis MJ, Knize AM, Coppola AR, Simon GM, Ngo N, Herbst RA, Herbst DM, Reed AW et al.. (2018) Monoacylglycerol Lipase Inhibition in Human and Rodent Systems Supports Clinical Evaluation of Endocannabinoid Modulators. J Pharmacol Exp Ther, 367 (3): 494-508. [PMID:30305428]
5. Ghafouri N, Tiger G, Razdan RK, Mahadevan A, Pertwee RG, Martin BR, Fowler CJ. (2004) Inhibition of monoacylglycerol lipase and fatty acid amide hydrolase by analogues of 2-arachidonoylglycerol. Br J Pharmacol, 143 (6): 774-84. [PMID:15492019]
6. Granchi C, Rizzolio F, Bordoni V, Caligiuri I, Manera C, Macchia M, Minutolo F, Martinelli A, Giordano A, Tuccinardi T. (2016) 4-Aryliden-2-methyloxazol-5(4H)-one as a new scaffold for selective reversible MAGL inhibitors. J Enzyme Inhib Med Chem, 31 (1): 137-46. [PMID:25669350]
7. Ikeda S, Sugiyama H, Tokuhara H, Murakami M, Nakamura M, Oguro Y, Aida J, Morishita N, Sogabe S, Dougan DR et al.. (2021) Design and Synthesis of Novel Spiro Derivatives as Potent and Reversible Monoacylglycerol Lipase (MAGL) Inhibitors: Bioisosteric Transformation from 3-Oxo-3,4-dihydro-2H-benzo[b][1,4]oxazin-6-yl Moiety. J Med Chem, 64 (15): 11014-11044. [PMID:34328319]
8. Jiang M, Huizenga MCW, Wirt JL, Paloczi J, Amedi A, van den Berg RJBHN, Benz J, Collin L, Deng H, Di X et al.. (2023) A monoacylglycerol lipase inhibitor showing therapeutic efficacy in mice without central side effects or dependence. Nat Commun, 14 (1): 8039. [PMID:38052772]
9. Long JZ, Nomura DK, Vann RE, Walentiny DM, Booker L, Jin X, Burston JJ, Sim-Selley LJ, Lichtman AH, Wiley JL et al.. (2009) Dual blockade of FAAH and MAGL identifies behavioral processes regulated by endocannabinoid crosstalk in vivo. Proc Natl Acad Sci USA, 106 (48): 20270-5. [PMID:19918051]
10. Niphakis MJ, Cognetta 3rd AB, Chang JW, Buczynski MW, Parsons LH, Byrne F, Burston JJ, Chapman V, Cravatt BF. (2013) Evaluation of NHS carbamates as a potent and selective class of endocannabinoid hydrolase inhibitors. ACS Chem Neurosci, 4 (9): 1322-32. [PMID:23731016]
11. Niphakis MJ, Johnson DS, Ballard TE, Stiff C, Cravatt BF. (2012) O-hydroxyacetamide carbamates as a highly potent and selective class of endocannabinoid hydrolase inhibitors. ACS Chem Neurosci, 3 (5): 418-26. [PMID:22860211]
12. Petersen A, Benz J, Grether U, Hornsperger B, Kocer B, Kuhn B, Richter H, Tsuchiya S, Qui Y, Chen R. (2019) Octahydropyrido[1,2-alpha]pyrazines as magl inhibitors. Patent number: WO2019134985A1. Assignee: Hoffmann-La Roche. Priority date: 08/01/2018. Publication date: 11/07/2019.
13. Wyatt RM, Fraser I, Welty N, Lord B, Wennerholm M, Sutton S, Ameriks MK, Dugovic C, Yun S, White A et al.. (2020) Pharmacologic Characterization of JNJ-42226314, [1-(4-Fluorophenyl)indol-5-yl]-[3-[4-(thiazole-2-carbonyl)piperazin-1-yl]azetidin-1-yl]methanone, a Reversible, Selective, and Potent Monoacylglycerol Lipase Inhibitor. J Pharmacol Exp Ther, 372 (3): 339-353. [PMID:31818916]
14. Xiong F, Ding X, Zhang H, Luo X, Chen K, Jiang H, Luo C, Xu H. (2021) Discovery of novel reversible monoacylglycerol lipase inhibitors via docking-based virtual screening. Bioorg Med Chem Lett, 41: 127986. [PMID:33766770]
15. Zhi Z, Zhang W, Yao J, Shang Y, Hao Q, Liu Z, Ren Y, Li J, Zhang G, Wang J. (2020) Discovery of Aryl Formyl Piperidine Derivatives as Potent, Reversible, and Selective Monoacylglycerol Lipase Inhibitors. J Med Chem, 63 (11): 5783-5796. [PMID:32429662]