GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
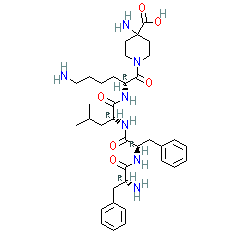
Synonyms: CR845 | D-Phe-D-Phe-D-Leu-D-Lys-[γ-(4-N-piperidinyl)amino carboxylic acid] | FE-202845 | Kapruvia® | Korsuva® | SEQ ID NO: 2 [6]
difelikefalin is an approved drug (FDA (2021), EMA (2022))
Compound class:
Peptide
Comment: Difelikefalin is a first-in-class, peripherally acting, highly selective peptide agonist of the κ-opioid receptor, that was developed for potential to treat chronic itch [4] and it is claimed in patent US7402564B1 [6]. This patent provides extensive results from preclinical testing, assessing the analgesic and anti-pruritic potential of difelikefalin.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Difelikefalin (with research code CR845) was evaluated in Phase 3 clinical trial as an intravenous (i.v.) analgesic to treat post abdominal surgery pain (see NCT02542384). NCT02229929 (Phase 2) assessed i.v. CR845 in hemodialysis patients suffering from uremic pruritus [1] and NCT02524197 (Phase 2) investigated orally administered CR845 in patients with hip or knee osteoarthritis. In August 2021, the FDA approved i.v. difelikefalin as a treatment for pateints with chronic kidney disease who develop hemodialysis-associated moderate-to-severe pruritus (uremic itch) [5], based on efficacy data arising from the KALM-1 and KALM-2 clinical trials. |
Mechanism Of Action and Pharmacodynamic Effects  |
| Difelikefalin is believed to activate κ receptors on peripheral nerve cells (thereby inhibiting afferent nerve activity and pain signalling) and on immune cells (inhibiting release of pro-inflammatory mediators such as cytokines and prostaglandins) [4]. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT02229929 | Safety and Pharmacokinetics of IV CR845 in Hemodialysis Patients, and Its Efficacy in Patients With Uremic Pruritus | Phase 2 Interventional | Cara Therapeutics, Inc. | ||
| NCT02524197 | A Study of the Safety and Effectiveness of Orally Administered CR845 in Patients With Osteoarthritis of the Hip or Knee | Phase 2 Interventional | Cara Therapeutics, Inc. | ||
| NCT02542384 | A Study Evaluating the Overall Pain Relief and Safety of Intravenous (IV) CR845 in Patients Undergoing Abdominal Surgery | Phase 2/Phase 3 Interventional | Cara Therapeutics, Inc. | ||
| NCT03636269 | CR845-CLIN3103: A Global Study to Evaluate the Safety and Efficacy of CR845 in Hemodialysis Patients With Moderate-to-Severe Pruritus | Phase 3 Interventional | Cara Therapeutics, Inc. | This study (KALM-2) was an international trial, whose results contributed to the FDA's first approval of difelikefalin. | 3 |
| NCT03422653 | A Study to Evaluate the Safety and Efficacy of CR845 in Hemodialysis Patients With Moderate-to-Severe Pruritus (KALM-1) | Phase 3 Interventional | Cara Therapeutics, Inc. | This study (KALM-1) was conducted in the United States, and its results were pivotal for difelikefalin's FDA approval. | 2 |