GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: BMS 986238 | BMS986238
Compound class:
Peptide
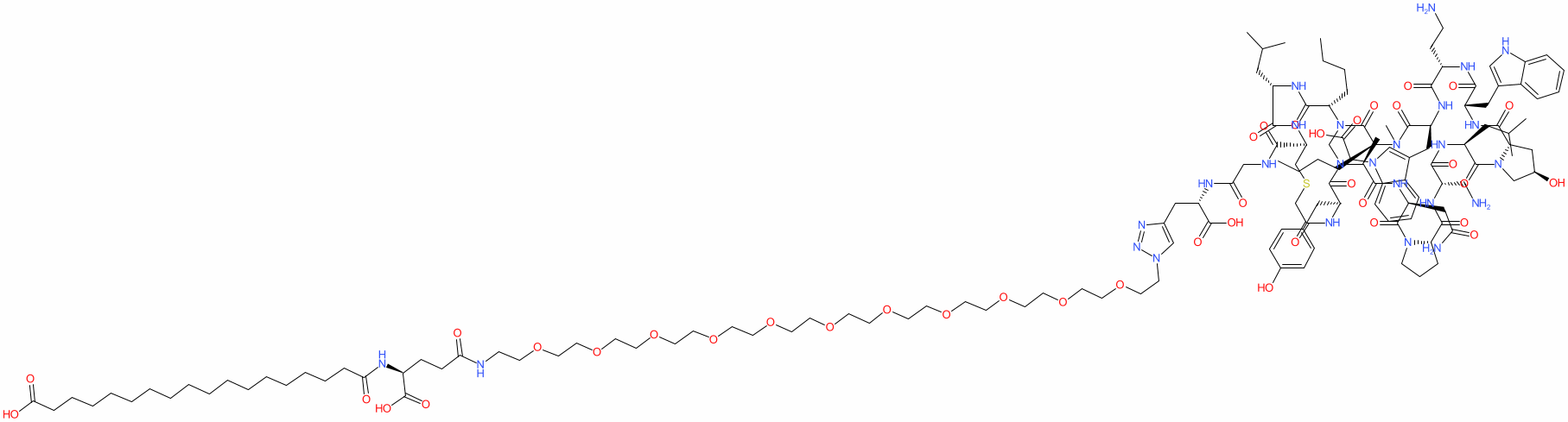
Comment: BMS-986238 is a macrocyclic peptide that inhibits programmed death-ligand 1 (PD-L1) [3]. It was designed as an alternative to anti-PD-L1 monoclonal antibodies and is a preclinical lead. Related structures are claimed in Bristol-Myers Squibb's patent WO2016077518A1 [1] with thioether peptide synthetic procedures described in [2].
|
|
|||||||||||||||||
Peptide Sequence  |
|
| Ac-Tyr-{NMe-Ala}-Asn-Pro-Dap-Leu-Hyp-Trp-Dab-Trp{CH2COOH}-{NMe-Nle}-(NMe-Nle)-Leu-Cys-Gly-DPra(azido-PEG11-γGlu-C18 diacid) | |
| Chemical Modification | |
| Thioether bridge between Tyr1 and Cys14 | |
Download 2D Structure  |
|
| Canonical SMILES | Download |
| Isomeric SMILES | Download |
| InChI standard identifier | Download |
| InChI standard key | Download |
Molecular structure representations generated using Open Babel