GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
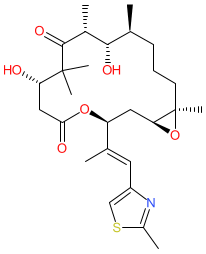
Synonyms: EPO-906 | EPO906 | patupilone
Compound class:
Natural product
Comment: Epothilone B is a macrolide from plant (Sorangium cellulosum, a soil-dwelling Gram-negative myxobacterium), Aspergillus fumigatus [3] and insect (Apis cerana, Asiatic honey bee) sources. It mimics the microtubule binding activity of taxoid drugs such as paclitaxel (taxol) [1,5], but is active in [4] paclitaxel resistant cells. It acts to stabilise microtubules, which induces mitotic arrest and cell death.
|
|
|||||||||||||||||||||||||||||||||||
Download 2D Structure  |
|
| Canonical SMILES | Download |
| Isomeric SMILES | Download |
| InChI standard identifier | Download |
| InChI standard key | Download |
Molecular structure representations generated using Open Babel